Super Cialis
Buy super cialis on line amex
The department may accept an evaluation report issued by the American college of radiology as evidence that a radiation machine erectile dysfunction psychological causes buy super cialis 80mg with amex, the personnel operating the radiation machine, and the facility in which the radiation machine is used meet those criteria. If at any time the department determines that it will not accept any evaluation reports issued by the American college of radiology as evidence that a radiation machine, the personnel operating the radiation machine, and the facility in which the radiation machine is used meet those criteria, the department shall promptly notify each person who has registered a radiation machine used exclusively to perform mammography under this part and the rules promulgated under this part. The physician designated under this subparagraph is responsible for conducting an on-site visit to each mammography station within the facility at least monthly for the purpose of providing professional feedback regarding clinical image quality and quality assurance procedures, for review of quality control documentation, and for ensuring that safe operating procedures are used in the delivery of mammographic services. If the physician designated under this subparagraph practices primarily outside of the facility, the physician shall keep a log of each on-site visit signed by the physician. The chief administrative officer of the facility or his or her designee may request to view the log at any time. The physician designated under this subparagraph shall meet the requirements of subdivision (g)(i) and (ii) or, until January 1, 1996, the requirements of subdivision (g)(ii) and (iii). Until the expiration of 2 years after the effective date of the amendatory act that added this subdivision, a physician or osteopathic physician licensed under article 15 who has been eligible for certification in radiology or diagnostic radiology for more than 2 years shall be considered to meet the requirement of this subparagraph. For purposes of this subparagraph, the department may accept time spent in a residency program that includes specific training in mammography if the individual has documentation of the residency program that is satisfactory to the department. A temporary authorization granted under this subsection after February 16, 1991 is effective for no more than 12 months. The department may withdraw a temporary authorization before its expiration if the radiation machine, the personnel operating the radiation machine, or the facility in which the radiation machine is used does not meet 1 or more of the standards set forth in subsection (2). A person who owns or leases more than 1 radiation machine used for mammography shall obtain authorization for each radiation machine. The department shall process and respond to an application within 30 days after the date of receipt of the application. Upon determining to grant mammography authorization for a radiation machine, the department shall issue a certificate of registration specifying mammography authorization for each authorized radiation machine. A mammography authorization is effective for 3 years contingent upon the radiation machine, the personnel operating the radiation machine, and the facility in which the radiation machine is operated for which the mammography authorization is issued meeting 1 of the following requirements: (a) Maintaining continued accreditation by the American college of radiology. After that initial inspection, the department shall annually inspect the radiation machine and may inspect the radiation machine more frequently. The facility shall post the certificate or other document near the inspected radiation machine. The department shall incorporate its findings in the order and shall provide an opportunity for a hearing within 5 working days after issuance of the order. An application for reinstatement of a mammography authorization shall be filed and processed in the same manner as an application for mammography authorization under subsection (4), except that the department shall not issue a reinstated certificate of mammography registration until the department receives the reinspection fee required under section 13522(5), inspects the radiation machine, and determines that it meets the standards set forth in subsection (2). The department shall conduct an inspection required under this subsection no later than 60 days after receiving a proper application for reinstatement of a mammography authorization. If a person continues to violate subsection (1) for a period of 2 weeks after a fine is imposed under this subsection, the department shall post a conspicuous notice on the unauthorized radiation machine and at the entry to the facility where the radiation machine is located warning the public that the facility is performing mammography using a radiation machine that is a substantial hazard to the public health. However, dense breast tissue can make it harder to find cancer through a mammogram. This information about the result of your mammogram is given to you to raise your awareness. Use this information to discuss with your health care provider whether other supplemental tests in addition to your mammogram may be appropriate for you, based on your individual risk. If you are self-referred, a report of your results was sent to you in addition to this summary. If, after the effective date of this section, new terms are defined in revised guidelines or systems for breast imaging reporting of mammography screening, and the department determines that those new terms are more appropriate for the purposes of the information required to be provided under this section, the department, by order, may update the definition of dense breast tissue under this subsection to use those new terms. Upon issuance, the department shall forward an order issued under this subsection to the legislature. A municipality or a department, agency, or official of a municipality may not license, regulate, or require the registration of a radioactive material or other source of ionizing radiation. An individual operating the system is not required to wear a lead apron or other personal monitoring equipment while operating the system if it is determined that the use of the system is in compliance with part 381 of the Michigan occupational safety and health administration occupational health standards, R 325. Upon request, a registrant shall make a lead apron or other personal monitoring equipment available to an individual who operates the system. The governor shall appoint, with the advice and consent of the senate, a radiation advisory board of 9 members, 3 of whom shall represent industry, 3 the healing arts, and 3 the public and private institutions of higher learning. The members shall be reimbursed for necessary and actual expenses incurred in attendance at meetings or for authorized business of the board pursuant to section 1216. The board shall furnish to the department technical advice the board deems desirable or the department may reasonably request on matters relating to the radiation control program. A person who violates this part or a rule promulgated under this part or who fails to obtain or comply with conditions of licensure or registration under this part is guilty of a misdemeanor, punishable by imprisonment for not more than 180 days, or a fine of not more than $10,000. If, after thorough investigation by the department, it is the judgment of the department that a person has engaged in or is about to engage in an act or practice which constitutes a violation of this part or a rule or order, the attorney general, at the request of the department, shall make application to the appropriate circuit court for an order enjoining the act or practice or for an order directing compliance with this part or a rule or order issued pursuant to this part. As used in this part, the words and phrases defined in sections 13702 to 13704 have the meanings ascribed to them in those sections. For transfer of powers and duties of radioactive materials program from department of health and human services to department of health and human services, see E. Processing does not include incineration or dilution of a material that has a radioactive concentration that is greater than the radioactive concentration of low-level radioactive waste. Remedial action includes, but is not limited to , actions at the location of the release such as storage, confinement, perimeter protection which may include dikes, trenches, and ditches, clay cover, neutralization, dredging or excavation, repair or replacement of leaking containers, collection of leachate and runoff, efforts to minimize the social and economic harm of processing, provision of alternative water supplies, and any required monitoring to assure that the actions taken are sufficient to protect the public health, safety, and welfare, and the environment. Subject to any limitations in this part, the department shall have the regulatory responsibility that is held by this state in all matters related to the generating, storage, processing, handling, transporting, possession, or disposal of waste. The governor with the advice and consent of the senate may enter into 1 or more agreements with the federal government negotiated pursuant to this subsection. In addition, the department and the attorney general shall submit written recommendations and the rationale supporting the recommendations to the legislature regarding whether this state should include naturally occurring or accelerator produced radioactive materials known as N. The recommendation required in this subsection shall be submitted by April 1, 1988. In fulfilling the requirement to promulgate rules, the director shall promulgate rules necessary to implement the provisions of this part that pertain to the issuance of permits to generators, transporters, collectors, and processors, including rules pertaining to the possession of waste by a generator, transporter, collector, or processor that is incidental to the regulated activity of the permit holder. Prior to approving a waiver under this subdivision, the director shall forward the proposed approval and supporting documentation to the department of natural resources for review and written comments. Except as otherwise provided in subsection (3), if this state has full agreement state status, a person shall not dispose of waste in this state except at the disposal site licensed by the director through the issuance of a construction and operating license under this part. Following the issuance of a construction and operating license under this part, the director with the written concurrence of the authority may grant or deny an application for a waiver of the requirement that waste be disposed of only in the disposal site if either of the following occurs: (a) If this state has obtained full agreement state status with the federal government, the department approves the disposal of the waste in a location other than the disposal site and concludes that the waiver will not harm the public health, safety, or welfare, or the environment and will not substantially impact on the volume of waste available for disposal in the disposal site or the financial solvency of the disposal site. In addition, if this state is a member of a compact the department shall assure that this state does not accept waste for disposal from any member of the compact that does either of the following: (a) Is delinquent in paying dues or fees payable under the compact. The minimum criteria shall reflect and shall be updated to include state-of-the-art technology in regard to disposal site design, construction, operation, and waste disposal technology. The criteria shall be developed and prepared in the form of specifications to be included in the construction and operating license issued to the authority pursuant to sections 13712 to 13714 and in any modification of that license. The criteria at a minimum shall comply with criteria adopted under the atomic energy act of 1954, 42 U. Acceptable disposal technologies shall be limited to above and below ground canisters or above and below ground vaults, or both. The criteria shall also include provisions for monitoring at the disposal site and within the disposal unit and provisions for the recoverability of waste that has been disposed of in the disposal site. The licensing requirements for the design, construction, and operation of the disposal site shall be at least as stringent as all applicable federal design, construction, and operating requirements. The director shall consider only an application submitted by the authority for a construction and operating license. However, the authority may submit a license that has been prepared for the authority pursuant to a contract entered into by the authority as provided in the low-level radioactive waste authority act. The director may waive all or any portion of this requirement for a person who is a corporation with publicly traded stock. If debt liability is held by a chartered lending institution, information required in this subsection shall not be required from that institution. The authority shall provide the department with the information required in subsection (1) for persons with whom the authority enters into contracts following the original submittal of an application for a construction and operating license. The authority shall file as a part of the application for a construction and operating license a surety bond, secured trust fund, or other suitable secured instrument or mechanism that shall be approved by the department and shall cover the cost of site closure and stabilization.

80 mg super cialis mastercard
Common area includes low libido erectile dysfunction treatment discount super cialis 80 mg visa, but is not limited to , a hallway, a stairway, a laundry and recreational room, a playground, a community center, a garage, and a boundary fence. Component or building component, includes but is not limited to , a specific interior or exterior design or structural element or fixture. For purposes of case management of children 6 years of age or less, elevated blood level means an excessive absorption of lead that is a confirmed concentration of lead in whole blood of 10 ug/dl. This part requires that all lead-based paint activities be performed by certified individuals and persons, except for those circumstances and persons described in section 5453(2). A person conducting a lead-based paint activity shall comply with the standards for performing lead-based paint activities contained in this part and the rules promulgated under this part. A person providing an accredited training program shall comply with the standards for accreditation and training certification prescribed in this part and the rules promulgated under this part. In the case of approval, the department shall send a certificate of accreditation to the applicant. Before disapproving an application, the department may advise the applicant as to specific inadequacies in the application for accreditation or specific instances where the training program does not meet the requirements of this part or the rules promulgated under this part, or both. The department may request additional information or materials from the training program under this section. A training manager may designate guest instructors as needed to provide instruction specific to the lecture, hands-on activities, or work practice components of a course. The department shall promulgate rules to determine the minimum curriculum requirements for the inspector course. The department shall promulgate rules to determine the minimum curriculum requirements for the risk assessor course. The department shall promulgate rules to determine the minimum curriculum requirements for the supervisor course. The department shall promulgate rules to determine the minimum curriculum requirements for the project designer course. The department shall promulgate rules to determine the minimum curriculum requirements for the abatement worker course. The department shall promulgate rules to determine the minimum curriculum requirements for the clearance technician course. Each individual enrolled in the training program must successfully complete the hands-on skills assessment, if conducted for that course, and receive a passing score on the course test in order to pass a course. The course completion certificates shall include: (a) the name and address of the individual, along with a unique identification number. The quality control plan shall contain at least both of the following elements: (a) Procedures for periodic revision of training materials and the course test to reflect innovations in the field. The work practice standards shall be taught in the appropriate courses to provide trainees with the knowledge needed to perform the lead-based paint activities. A training program shall meet those minimum requirements contained in rules promulgated by the department in order to obtain department accreditation. Upon approval, the department shall send a certificate of accreditation to the applicant. Before disapproval, the department may advise the applicant as to specific inadequacies in the application for accreditation or specific instances where the continuing education course does not meet the requirements of this part and the rules promulgated under this part, or both. The department may also request additional information or materials retained by the training program. To maintain certification, an individual must be recertified pursuant to this part. An individual is not eligible to take the third party exam more than 3 times within the 6 months after receiving a course completion certificate. An individual who does not pass the third party exam after 3 attempts shall repeat the appropriate course from an accredited training program in order to be eligible to retake the exam. To maintain certification, the individual must be periodically recertified pursuant to this part. Within that time period, the department shall respond with either a certificate of approval or a letter describing the reasons for a disapproval. Beginning on March 1, 1999, all lead-based paint activities shall be performed by an individual certified in the appropriate discipline under this part and pursuant to the work practice standards prescribed in rules promulgated by the department. The increased fees shall be used by the department as the basis for calculating fee increases in subsequent fiscal years. Before beginning a lead-based paint abatement, a person conducting lead-based paint abatement shall notify the department, on forms provided by the department or through electronic methods approved by the department, regarding information the department considers necessary in order to conduct an unannounced site inspection. The person shall send notification not less than 3 business days before commencing the lead-based paint abatement. The legislature shall annually appropriate to the department an amount sufficient to administer and enforce this part. These funds shall be offset by funds received from federal agencies in the form of grants or other funding provisions. All funds generated by this part shall be deposited into the general fund to be used exclusively by the department to carry out the duties and responsibilities of this part. Such activities include, but are not limited to , unannounced inspections of lead abatement project sites. In making its recommendations, the department shall consult with affected stakeholders and shall consider the effects of those maintenance practices on the availability and affordability of housing and credit. As part of this system, the department shall require that results of all blood lead level tests conducted in Michigan be reported to the department as provided for in rule and that when the department receives notice of blood lead levels above 10 micrograms per deciliter, it shall initiate contact with the local public health department or the physician, or both, of the child whose blood lead level exceeds 10 micrograms per deciliter. The report shall compare these rates with those of previous fiscal years and the department shall recommend methods for improving compliance with guidelines issued by the federal centers for disease control and prevention, including any necessary legislation or appropriations. The report shall be given to the appropriate committees of the legislature and be made available to the general public upon request. The form shall include, at a minimum, the following: (a) Name of the owner of the building. A person who wishes to register under this subsection shall execute and return the registration form to the department with payment of the registration fee in an amount as prescribed by the department. The department may charge a reasonable, cost-based fee for providing copies of the lead safe housing registry under this subsection. The legislature further recognizes the need to educate the citizens of this state regarding those threats. The department may deny, suspend, or revoke certification or accreditation issued under this part if a certified person, accredited training program, certified individual, or a person allegedly engaged in lead-based paint activity is found to be not in compliance with this part or the rules promulgated under this part. In addition, the department may deny, suspend, or revoke a certification or accreditation issued under this part for 1 or more of the following: (a) Willful or negligent acts that cause a person to be exposed to a lead-containing substance in violation of this part, the rules promulgated under this part, or other state or federal law pertaining to the public health and safety aspects of lead abatement. A violation of this subsection may be prosecuted by either the attorney general or the prosecuting attorney of the judicial district in which the violation was committed. As used in this part: (a) "Children" means individuals who are 7 years old or younger. The department shall post on its website information about the hazards of lead-bearing substances and any programs it offers designed to educate individuals about those hazards. As used in this part: (a) "Child care article" means a product designed or intended by the manufacturer to facilitate the sleep, relaxation, or feeding of children or to help children with sucking or teething. In developing the state plan, consideration shall be given to all of the following: (a) A center shall be located so as to minimize transportation problems for patients and their families. The network shall include individuals qualified to perform all of the following tasks: (a) Provide information to , and obtain consent from, an affected individual or his or her family as provided in section 5529. The department shall identify 1 or more tissue repositories for the receipt and storage of tissue of affected individuals who are deceased. The department may identify an existing public or private facility or institution that is equipped to provide for storage of the tissue. If an affected individual or his or her family representative requests an autopsy, a network representative shall provide to that person information concerning the cost, purposes, and benefits of an autopsy, and the benefits of using the tissue for medical research and education. The network representative shall also request that the affected individual or his or her family representative sign a written consent to the autopsy, and a separate written consent to use of the tissue for medical research and education. The chronic disease advisory committee shall oversee the implementation of sections 5523 to 5539.
Diseases
- Choanal atresia deafness cardiac defects dysmorphia
- Myopathy, myotubular
- Kantaputra Gorlin syndrome
- Hypocalcemia, autosomal dominant
- Groll Hirschowitz syndrome
- Congenital ichtyosiform erythroderma
- Berdon syndrome
- Facial paralysis
Purchase super cialis canada
Adding zinc to prenatal iron and folate tablets improves fetal neurobehavioral develop ment erectile dysfunction drugs free sample discount super cialis online american express. Size of the zinc pools that exchange rapidly with plasma zinc in humans: Alternative techniques for measuring and relation to dietary zinc intake. Effect of dietary zinc on whole body surface loss of zinc: Impact on estimation of zinc retention by balance method. Effect of oral folic acid supplements on zinc, copper, and iron absorption and excretion. Dietary zinc intake and zinc concentrations of plasma, erythrocytes, and breast milk in antepartum and postpartum lactating and nonlactating women: A longitudinal study. Use of Vitamin and Mineral Supplements in the United States: Current Users, Types of Products, and Nutrients. Mild to moderate zinc deficiency in short children: Effect of zinc supplemen tation on linear growth velocity. Plasma and erythrocyte zinc concentrations and their relationship to dietary zinc intake and zinc supplementation during pregnancy in low-income African-American women. Zinc levels in maternal milk: the influence of nutritional status with respect to zinc during the third trimester of pregnancy. Serum extracellular superoxide dismutase activity as an indicator of zinc status in humans. Dietary intake and biochemical indices of nutri tional status in an elderly population, with estimates of the precision of the 7 d food record. Utilization of zinc from picolinic or citric acid complexes in relation to dietary protein source in rats. Erythrocytes, erythrocyte mem branes, neutrophils and platelets as biopsy materials for the assessment of zinc status in humans. The effect of zinc supplements on plasma zinc and copper levels and the reported symptoms in healthy volunteers. Effects of repletion with zinc and other micronutrients on neuro psychologic performance and growth of Chinese children. Low zinc intake during pregnancy: Its association with preterm and very preterm delivery. Effect of dietary picolinic acid on the metabolism of exogenous and endogenous zinc in the rat. Zinc and immune function: the biological basis of altered resistance to infection. Zinc ab sorption and intestinal losses of endogenous zinc in young Chinese women with marginal zinc intakes. Food consumption patterns of Canadian preschool children in relation to zinc and growth status. Effect of dietary calcium and phosphorus levels on the utilization of iron, copper, and zinc by adult males. Trace element status in healthy subjects switching from a mixed to a lactovegetarian diet for 12 months. Metallothionein expression is increased in monocytes and erythrocytes of young men during zinc supplementation. Homeostatic regulation of zinc absorption and endogenous losses in zinc-deprived men. Energy, protein, zinc and copper status of twenty-one elderly inpatients: Analysed dietary intake and biochemical indices. Use of enriched stable isotopes to determine zinc and iron absorption in elderly men. A stable isotope study of zinc absorption in young men: Effects of phytate and alpha-cellulose. A stable-isotope study of zinc, copper, and iron absorption and reten tion by young women fed vitamin B-6-deficient diets. Biochemical evidence suggestive of suboptimal zinc and vitamin A status in schoolchildren in northeast Thailand. Zinc supplemen tation and stunted infants in Ethiopia: A randomised controlled trial. Effect of low zinc intakes on basal metabolic rate, thyroid hormones and protein utilization in adult men. Zinc supplementation in infants with a nutritional pattern of failure to thrive: A double-blind, controlled study. Iron, copper, and zinc status: Re sponse to supplementation with zinc or zinc and iron in adult females. Iron and zinc nutriture of premeno pausal women: Associations of diet with serum ferritin and plasma zinc dis appearance and of serum ferritin with plasma zinc and plasma zinc disappear ance. Flux of intracellular labile zinc during apoptosis (gene-directed cell death) revealed by a specific chemical probe, Zinquin. Hepatic metallothionein as a source of zinc and cysteine during the first year of life. Environ mental Protection Agency, Office of Solid Waste, by Research Triangle Insti tute and American Biogenics Corporation under contract 68-01-7075. Enzymatic methylation of arsenic species and other new approaches to arsenic toxicity. Dietary Boron Modifies the Effects of Vitamin D Nutriture on Energy Metabolism and Bone Morphology in the Chick. Effects of vanadyl sulfate on carbohydrate and lipid metabolism in patients with non-insulin-dependent diabetes mellitus. Pathologic changes in rats and dogs from two-year feeding of sodium arsenite or sodium arsenate. Biochemical and morphological changes associated with long bone abnormalities in silicon deficiency. Cardiovascular system and kidney as specific targets of chronic exposure to vanadate in the rat: Functional and morphological findings. Studies in human lactation 3: Molybdenum and nickel in human milk during the first month of lactation. Arsenic carcinogenesis in animals and in humans: Mecha nistic, experimental, and epidemiological evidence. Cancer potential in liver, lung, blad der and kidney due to ingested inorganic arsenic in drinking water. Fulmi nant malignant arrythmia and multiorgan failure in acute arsenic poisoning. Oral vanadyl sulfate improves hepatic and peripheral insulin sensitivity in pa tients with non-insulin-dependent diabetes mellitus. Inorganic boron health effects in humans: An aid to risk assessment and clinical judgment. Survey of arsenic in total diet food composites and estimation of the dietary intake of arsenic by Canadian adults and children. One-year treatment of non-diabetic and streptozotocin diabetic rats with vanadyl sulphate did not alter blood pressure or haemato logical indices. Further characterization of the posttranslational modifications of core histones in response to heat and arsenite stress in Drosophila. Toxicity and carcinogenicity studies of boric acid in male and female B6C3F1 mice. Effects of vanadium on reproduction, gestation, parturition and lactation in rats upon oral adminis tration. Oral vanadium administration to streptozotocin-diabetic rats has marked negative side-effects which are independent of the form of vanadium used. Boron tolerable intake: Re-evaluation of toxicokinetics for data-derived uncertainty factors. Special Report on Ingested Inorganic Arsenic: Skin Cancer; Nutritional Essentiality. National primary drinking water regulations; Arsenic and clarifications to compliance and new source contaminants monitoring; Proposed rule. Oral vanadyl sulphate does not affect blood cells, viscosity or biochemistry in humans.
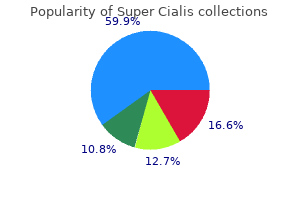
Discount super cialis 80 mg with mastercard
To capitalize more fully on this burgeoning interest erectile dysfunction pills amazon buy super cialis 80 mg mastercard, these disciplines need more precise informa tion on hazards to fetal development, symptomatology and treatment potentials for specific diagnostic conditions, and the values and limita tions of psychological test measurements. Furthermore, to keep abreast of new discoveries and program developments, these disciplines must share a terminology and language that permit communication. Our failure in this latter area has seriously handicapped efforts of profes sionals from different countries to learn from one another. The World Health Organization, mindful of these deficiencies and of our increasing fund of knowledge, has embarked upon a series of seminars to develop an international scheme for the diagnosis, classifi cation, and reporting of statistics in psychiatric disorders, including mental retardation. Com parative data among countries on the incidence and prevalence of mental retardation and the factors with which specific conditions are associated are not highly reliable. Although there are significant varia tions in prenatal care, population homogeneity, disease control, degree of environmental deprivation, and other factors causative or contributo ry to mental retardation, reported statistical differences may be more artifactual than real. Differences in the definition and conceptualization of mental retardation, inadequacies and variations in classification schemes used, confusion of terminology, and cultural variability in demands and expectations for human performance are only a few of the artifacts that preclude valid comparisons. Within and among countries, meaningful planning for the retarded cannot be accomplished until these issues are resolved. The 1969 seminar, cosponsored by the World Health Organization and the National Institute of Child Health and Human Development, was a milestone in the realization of these goals. It is clear that the complex issues confronted will require continuing attention, but mean ingful dialogue has begun and a sounder base for assessing the extent and diversity of this problem is being established. The study of the process of psychiatric and the recommendations made for the diagnosis, as provided by the diagnostic ex forthcoming ninth revision of the Interna ercise, should be used as the basis for under tional Classification of Diseases. In general standing the different schemes that psychia the Seminar agreed that the current classifi trists of different schools employ. Major cation of mental retardation is inadequate sources of variation and error should be and that a multiaxial scheme should be identified in order to improve the reliability adopted. In view of the variety of theoretical etiological factors, and 3) clinical psychiatric concepts regarding the etiology and path system, and would require that each axis be ogenesis of mental disorders, and because recorded. Information that should be of the paucity of evidence that might lead to a considered in classifying mental retardation choice between theories, emphasis should be includes: degree, organic aspects, psychiatric placed on the use of solid clinical facts as a and behavioral aspects, and psychosocial as starting point in developing a classification. In the development of a appendix 1): 1) Diagnostic exercises using classification of mental disorders, four prin case histories from previous seminars were carried out in Bulgaria, Czechoslovakia, Ja pan, and the U. Diagnostic Exercises the procedure, which had been successfully used in previous seminars, of beginning with a detailed discussion of problems arising from appreciated, and there was general comment the analysis of case histories and of video throughout the meeting on the outstandingly tapes, was again used in this one. Here the good choice of cases that highlighted the chief purpose was to enable participants first, to issues to be considered during the discussions. The purpose of tion of specific problems to approach general this exercise was, again, to illustrate different principles of diagnosis, classification, and facets of the diagnostic problem by showing a statistics in this area. The three patients shown illustrated problems of the differential Case History Exercise diagnosis of developmental disorders and Several months before the meeting partic mental retardation, the problem of the dif ipants received case histories of 11 patients ferential diagnosis of child psychosis and that they were asked to read and to analyze. Participants some who were typical and others who were were then given a case history and were borderline or had an uncertain diagnosis that invited to ask for additional information that posed considerable problems. It was chological evaluation, and some information thought, however, that the discussion would about his hospital course or disposal. Partic have been more fruitful had the videotapes ipants were invited to make a diagnosis of been available for replay during the discus the patient, using their own terminology, to sion, and it was recommended that arrange indicate whether the diagnosis was firm or ments be made for this in future seminars. Summaries of the data indicat Principal Topics of Discussion ing diagnostic agreement and disagreement Agreement on Diagnosis and Classification on the patients, as well as the comparison of diagnoses, were handed out at the meeting Level of intellectual retardation. For each and participants brought with them the case of the patients in the case history exercise, the material they had been sent. The excellent participants were asked to categorize the preparation of case histories by the U. The only clear instruction as to whether the same patient over whom there was appreciable dis condition should be recorded under one or agreement was a six-week-old boy with both headings. It was agreed most important to record the biological dis that it was not possible to make a meaningful orders underlying mental retardation, but assessment of intelligence in infancy and that dissatisfaction was expressed with the fourth judgments on level of retardation in very digit coding in that it necessitated judgments young children could only be approximate. This issue was tion existed for individuals from minority returned to later in the Seminar. Whereas patients showed disorders of behavior, as well intelligence tests are of great value in assess as mental retardation. For example, the third ing level of retardation, they should never be patient in the case history exercise was a hos used in isolation from clinical considerations tile, extremely hyperactive child who had of social-adaptive functioning. When started several fires and who had gotten into individuals come from cultures other than frequent fights. Most of the partici rise to no difficulties in patients who had pants diagnosed psychosis of some type, but a clearly defined disease or disorder. This was It was generally agreed that this state of due to uncertainties about etiology and to the affairs was most unsatisfactory. In spite of a problems of multiple factors in etiology; thus high level of agreement on diagnosis, there different participants coded different etio was a very low level of agreement on coding. For example, in case one, a child this arose through three factors: 1) no satis with a definite neurological disorder of an factory means of coding child psychiatric or unknown type that had been present since behavior disorders; 2) no instruction on how birth, codings of. Several of the of mental retardation, even those due to or patients in both the case history and in the ganic brain dysfunction, are not associated videotape exercises showed a severe delay in with clearly diagnosable diseases, and 2) the the development of speech and/or language fourth digit of the mental retardation coding that could not be accounted for in terms of mental retardation. For example, the second was "intellectual level," and the third was patient in the videotape exercise was a five "associated and etiological factors. However, even needed for mental retardation but that some those participants who made a primary modifications might be required. Thus, at a minimum, it was essential case also emphasized the lack of instructions that the degree of retardation, as well as the on how to deal with multiple diagnoses. In discussing the case histories it was noted It was decided that this problem could be several times that the same problems were solved by either a multiaxial scheme, as being encountered as those previously dis proposed at the Paris Seminar, or by a mul cussed at the Paris Seminar on child psy ticategory scheme. It was decided decided that any scheme for the inclusion of that at least three major axes or categories such disorders must be simple and practical, were required, namely, those outlined in the must include only a basic minimum of Paris Seminar. For all mentally retarded pa information (in this respect classification tients the intellectual level, the associated or necessarily differed from both a diagnostic etiological factors, and the clinical psychiat formulation and nomenclature), and must ric syndrome would need to be recorded. The essential feature of the scheme scheme of classification in which all three is that for each patient three categories of axes had to be coded. Codings "clinical psychiatric syndrome," the second should be available that note where informa tion is not known or where no abnormality is our environment and our development; present. It tion would be regarded as present, and 3) the recommended that its suggestions on classi subdivision into degrees of retardation. In view of these concept of mental retardation, in that at least considerations it was agreed that, in line with 16 percent of the general population would be the recommendations of the Paris Seminar, considered retarded. The Committee also mental retardation should be assessed on the expressed itself as being strongly opposed to basis of current level of functioning without this expansion of the concept, taking the view regard to its nature or causation. When used appropriately, intelli should constitute only a guide, it always being necessary to take clinical considerations of gence tests could provide valuable guidelines social and adaptive functioning into account. Instead, the constitute a clearly defined disease of a rec terms should be carefully defined in the ognized type. A working party needs to be Manual on Psychiatric Disorders and Classi set up to determine how this should best be fications. The Seminar recommended current level of intellectual functioning can that the terms describing conditions com not be assessed either by standardized tests monly found and reported in the classifica or by clinical judgments. For ex because it demands a knowledge of the etiol ample, one of the patients in the exercise had ogy of the retardation, which is often lacking diabetes, as well as epilepsy, but only a small due to the pathogenesis of mental retardation number of the participants coded diabetes. Problems of intellectual retardation arise not infrequently in relation to psy chosocial factors, and the Seminar considered it desirable that there be provisions for the coding of such factors. The provision and definition of categories of psychosocial influences posed difficulties beyond the scope account of disorders in children, neurotic of the present Seminar, but it was recom disorders, personality disorders, psy 4 mended that a working party be set up to chosomatic disorders, and other clinical develop appropriate definitions for psy syndromes could be included in existing cod chosocial factors, both those important in the ings. By redefining psychoses and by provid pathogenesis of mental retardation and also ing extra digits, child psychoses could also be those influences, familial and other than fa included under the current codings. Normal milial, that are important in the pathogenesis variation, conduct disorder, and manifesta of emotional and behavioral disorders. In tion of mental subnormality only would need view of the importance of psychosocial additional codings. Adaptation reaction influences it was recommended that this be a would need an extra coding, but this might be separate axis or category to be recorded for provided by a redefinition and reorganization all patients instead of the present fourth digit, of category 307, "transient situational dis-.
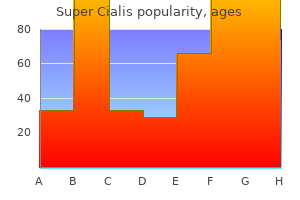
Generic super cialis 80mg visa
Members and their physicians are informed by mail of potential gaps and are instructed on how to improve adherence to existing therapies erectile dysfunction doctor houston buy super cialis cheap. The program allows you to get a discount on many prescription drugs not covered by our prescription benefit. Enhanced CaremarkDirect Retail is a value-added program that provides you with safe, convenient access to competitively priced, non-covered prescriptions, and certain over the-counter drugs. Flexible benefits option Under the flexible benefits option, we determine the most effective way to provide services. If we identify a less costly alternative, we will ask you to sign an alternative benefits agreement that will include all of the following terms in addition to other terms as necessary. Your Health Assessment profile provides information to put you on a path to good physical health. If you have Self Only coverage with our Plan, when you complete the Health Assessment, we will enroll you in the CignaPlus Savings discount dental program and pay the Self Only CignaPlus Savings discount dental premium for the remainder of the calendar year in which you completed the Health Assessment provided you remain enrolled in our Plan. If you have Self Plus One or Self and Family coverage with our Plan, when at least two family members complete the Health Assessment, we will enroll you and your covered family members in the CignaPlus Savings discount dental program and pay the family CignaPlus Savings discount dental premium for the remainder of the year in which both Health Assessments were completed provided you remain enrolled in our Plan. You will speak to a pregnancy specialist and receive unlimited coaching calls to provide you with caring support to optimize your chances of having a healthy, full-term pregnancy. Healthy Pregnancies, Healthy Babies will work together with you and your doctor to develop a plan of care. Call 855-511-1893 to enroll in the Healthy Pregnancies, Healthy Babies program as soon as you know you are pregnant. Healthy Rewards A program available to all members that provides discounts on services that are not Program usually covered by the Plan. Virtual visits can be used for adults or children with minor acute non emergency medical conditions. Weight Management the Cigna Healthy Steps to Weight Loss Weight Management Program guides each Program person in creating their own tailored healthy living plan to help them eat right, participate in regular physical activity, and adopt habits that will lead to a healthy weight for life. The program is a non-diet approach to weight loss with an emphasis on changing habits. Each person seeking assistance with behavior change responds to treatment options in his or her own unique way. Participants, with the guidance of a Wellness Coach, a trained health professional, may select the online mode or the telephone coaching model. A toolkit is sent to each coaching program participant to assist him or her in achieving their plan goals. CignaPlus Savings (discount dental program) CignaPlus Savings is a discount dental program that provides members access to discounted fees with participating dental providers. This is a discount program and not insurance, and the member must pay the entire discounted charge for dental services. Hospital Plus (hospital indemnity) Hospital Plus is a hospital indemnity policy available for purchase from the United States Letter Carriers Mutual Benefit Association. This policy may be purchased throughout the year and is not subject to the health benefit plan open season. Hospital Plus means money in your pocket when you are hospitalized, from the first day of your stay up to one full year. Benefits will be based on the number of days in the hospital, up to 365 days or as much as $36,500 (if a $100 a day benefit is chosen). Use your benefits to pay for travel to and from the hospital, childcare, medical costs not covered by health insurance, legal fees, or other costs. Hospital Plus is renewable for life and you may keep your policy for as long as you like, regardless of benefits you have received or future health conditions. For more information and current benefits, please call the United States Letter Carriers Mutual Benefit Association at 202-638-4318 Monday through Friday, 8:00 a. The annual associate membership dues is in addition to your bi-weekly (or monthly) share of the health benefit premium. If you are a Postal Service employee, your regular membership dues are paid through authorized payroll deduction. Although we may list a specific service as a benefit, we will not cover it unless we determine it is medically necessary to prevent, diagnose, or treat your illness, disease, injury, or condition. For information on obtaining prior approval for specific services, such as transplants, see Section 3. Filing a Claim for Covered Services this Section primarily deals with post-service claims (claims for services, drugs or supplies you have already received). See Section 3 for information on pre-service claims procedures (services, drugs or supplies requiring prior Plan approval), including urgent care claims procedures. Consumer Driven Health Plan and Value Option: To obtain claim forms, claims filing advice or answers about our benefits, contact Cigna at 855-511-1893, or visit our website at When Medicare is not the primary payor, claims should be submitted directly to Cigna at the address shown on the reverse side of your identification card. Coordinating Benefits with Medicare and Other Coverage the Original Medicare Plan (Part A or Part B). Note: To file a mental health and substance use disorder treatment claim, see Section 5(e). Note: A clean claim is a claim which contains all necessary information for payment including any substantiating documentation. Clean claims do not require special handling or investigation prior to adjudication. Post-service claims We will notify you of our decision within 30 days after we receive your post-service procedures claim. If matters beyond our control require an extension of time, we may take up to an additional 15 days for review and we will notify you before the expiration of the original 30-day period. If you do not agree with our initial decision, you may ask us to review it by following the disputed claims process detailed in Section 8 of this brochure. Deadline for filing your Send us all the documents for your claim as soon as possible. You must submit the claim claim by December 31 of the year after the year you received the service. If you could not file on time because of Government administrative operations or legal incapacity, you must submit your claim as soon as reasonably possible. Once we pay benefits, there is a three year limitation on the reissuance of uncashed checks. We may delay processing information or deny benefits for your claim if you do not respond. The Plan, its medical staff and/or an independent medical review determines whether services, supplies and charges meet the coverage requirements of the Plan (subject to the disputed claims procedure described in Section 8. We are entitled to obtain medical or other information including an independent medical examination that we feel is necessary to determine whether a service or supply is covered. Authorized You may designate an authorized representative to act on your behalf for filing a claim or Representative to appeal claims decisions to us. For urgent care claims, a health care professional with knowledge of your medical condition will be permitted to act as your authorized representative without your express consent. Notice Requirements the Secretary of Health and Human Services has identified counties where at least 10 percent of the population is literate only in certain non-English languages. The non English languages meeting this threshold in certain counties are Spanish, Chinese, Navajo and Tagalog. Any notice of an adverse benefit determination or correspondence from us confirming an adverse benefit determination will include information sufficient to identify the claim involved (including the date of service, the health care provider, and the claim amount, if applicable), and a statement describing the availability, upon request, of the diagnosis and procedure codes and its corresponding meaning, and the treatment code and its corresponding meaning. Please follow this Federal Employees Health Benefits Program disputed claims process if you disagree with our decision on your post-service claim (a claim where services, drugs or supplies have already been provided). If you disagree with our pre-service claim decision, we describe the process you need to follow if you have a claim for services, drugs or supplies that must have prior Plan approval, such as inpatient hospital admissions. However, our failure to provide you with new evidence or rationale in sufficient time to allow you to timely respond shall not invalidate our decision on reconsideration. In the case of a post-service claim, we have 30 days from the date we receive your request to: 2 a) Pay the claim; or b) Write to you and maintain our denial; or c) Ask you or your provider for more information.
Syndromes
- Widen (dilate) an airway that is blocked or narrowed
- Bruising or bleeding that occurs more easily
- Cibalith
- Blocked airway from swollen tonsils
- Both vaccines protect against the two types of HPV that cause most cases of cervical cancer. Other, less common types of HPV can still cause cervical cancer.
- Giving magnesium or potassium through a vein
- Mediastinitis
- Close your heart and take you off the heart-lung machine.
- Loss of bladder or bowel control
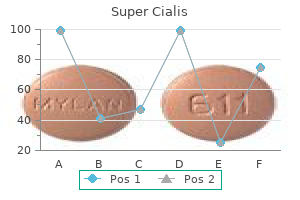
Purchase cheap super cialis on-line
Organized systems of care have the potential to create informational databases that can be readily accessed in crisis situations erectile dysfunction hypertension drugs generic 80mg super cialis mastercard. However, increased information sharing requires a heightened sensitivity that these are privileged documents, and the clinician should participate in safeguarding them against potential misuse. Because of these differences, culturally diverse children and their families have many specific mental health needs relevant to assessment, treatment approaches and modalities, and support services. This model also calls for the use of natural strengths and resources in diverse communities and cultures that are protective and support children and families dealing with emotional disturbance. There is beginning evidence that adopting such practices results in improved access to services and retention in treatment (Pumariega et al. The wraparound process as formally defined is most effectively applied in an organized system of care in which the focus of service planning is the child and family team with an assigned facilitator, and in which providers are encouraged to devote time to attending interagency meetings. Even in less developed or organized systems, however, elements and principles of the wraparound process can be incorporated. For example, use of a strength-based orientation and discussion of needs rather than problems promotes more active engagement of families in service planning activities. A number of studies of the wraparound process in different communities with diverse populations of at-risk children and families have reported positive outcomes in terms of reduction of externalizing behavioral problems, level of function, reduction in out-of-home placement, improved family management skills and function, and service recipient/family satisfaction (Burchard et al. The wraparound process is best suited for children and their families with complex mental health and related needs who have not benefited from traditional services. Wraparound as a process alone may not be effective if the specific interventions themselves are not effective or if the skills and training of clinicians providing care are not adequate. Therefore, interventions with the strongest evidence base should be prioritized in clinical and systems planning. Department of Health and Human Services, 1999; Burns and Hoagwood, 2002; Rogers, 2003). This includes both clear definitions of disciplinary roles and contributions as well as flexibility in these when necessary for the best outcomes for children and families. Potential roles for child and adolescent psychiatrists include not only direct service provision. It is important in such situations for physicians to explore mechanisms to broaden their involvement and add additional value to the agency processes. Examples might include training and consultation to other clinicians to improve intake and triage operations, supervising other medical professionals to expand the medical resource, assisting the agency or program in selecting the most appropriate evidence-based interventions for the population, and using telemedicine or videoconferencing to increase opportunities for participation in team processes. Physicians should advocate for funding for attending interdisciplinary meetings, especially for children with complex psychiatric and medical issues. It is important for the physician to advocate to be included in clinical and system planning meetings as part of the role when negotiating a position in a mental health or other child-serving agency. Growing awareness of the potential benefits of pharmacotherapy for children and adolescents has led to increased emphasis on the psychopharmacological role of the child and adolescent psychiatrist in community systems of care. This role is an important one, especially as newer and potentially more effective pharmacological agents continue to emerge. Ongoing management may be provided by the child and adolescent psychiatrist, or the child and adolescent psychiatrist may function as a consultant. Pharmacotherapy in systems of care should focus on functional improvement as well as on symptomatic relief. It should also include collaboration and psychiatric consultation around medication management with other prescribing medical professionals (Pumariega & Fallon, 2003). It is important that practitioners of pharmacotherapy not practice in isolation from the rest of the treatment team and treatment planning process. Lack of adequate contact of the children and families with the prescribing physician or medical practitioner often leads to children and families feeling uninformed, disempowered, and mistrustful of pharmacological therapies (Pumariega & Fallon, 2003). Prescribing physicians in systems of care should promote clinical standards for effective pharmacological therapy, including the use of evidence-based systematic assessment and symptom rating tools and the use of evidence-based pharmacological interventions. They should become actively involved in quality assurance and improvement around pharmacological decision-making, practices, and therapies. Informed consent must be obtained, ideally by the physician, but when this is not feasible at a minimum the physician should oversee the process and be available to answer questions of the parents or legal guardian. Attention should be given to cultural factors in pharmacotherapy, including consideration of ethnobiological factors, culturally appropriate decision-making and consent processes, and addressing issues of stigma and fears about the misuse of medications. The clinician should be familiar with the organizational context of the agency or system in which he/she is working in order to advocate effectively for adequacy of resources and practices to meet the needs of children and families served. Clinicians in systems of care should become familiar with agency and system administrative structures, mandates or contracted responsibilities, policies and procedures, and organizational culture. Clinicians should become involved in administrative and organizational processes as a means of advocacy for improved access and quality of care. Additionally, clinicians should be familiar with evidence-based community-based interventions and treatment modalities and advocate for their adoption within systems-of-care agencies and programs (Rogers, 2003). Clinicians should participate in quality assurance and improvement processes and the evaluation of agency and systems outcomes (Friesen and Winters, 2003). The system of care should be accountable for clinical outcomes and actively involved in quality improvement efforts. With increased societal demand for fiscal accountability, interest has grown in measuring outcomes for evaluation of individual mental health services and program effectiveness. Clinicians and health care administrators have also recognized that process is not by itself an adequate indicator of quality of care, and therefore clinical outcomes need to be measured. Community systems of care for children or youth with serious emotional and behavioral disorders have many stakeholders, including the child, family, school, mental health or other service agency, primary health care provider, funding agency, etc. Several models have been presented as ways of conceptualizing different domains of outcomes that might be measured. The system-of-care model entails accountability of the system for outcomes, also recognizing that functional outcomes may be as important to families as symptomatic improvement. Traditional services (and clinical research) have most often addressed symptomatic improvement and underemphasized functional issues more salient for day-to-day family life. A child or family dropping out of service should trigger review of the treatment plan rather than discharge from care. It is incumbent on the system (and clinicians working within it) to collaborate with families in deciding what the desired outcomes should be and share accountability with them for those outcomes. Children should have access to a continuum of care with assignment of level or intensity of care determined by clinically informed decision-making. Children should have access to a full continuum of services, with level or intensity of care determined by clinically informed decision-making rather than arbitrary protocols or benefit limitations. Family members hold true expertise on their own children and have a primary decision making role in treatment planning. As Burns, Hoagwood, and Mrazek have pointed out, It is becoming increasingly clear that family engagement is a key component not only of participation in care, but also in the effective implementation of it. Families should be respected as experts on their own children and enlisted as partners in the care of their children. There is a substantial amount of literature supporting parent involvement in service delivery. Within the system of care environment, the Child and Family Team concept has emerged as a model service planning/ treatment team. The family often has a trained advocate who has learned to navigate the system also involved with the planning and the team meetings. Roles of families and youth in organizational governance Families and youth bring valuable and insightful experiences to organizational governance and policy development.
Order on line super cialis
This section does not apply to a hospital licensed or operated by the department of mental health or the federal government or to a veterinary hospital impotence and diabetes generic 80mg super cialis otc. The owner, operator, and governing body of a hospital licensed under this article: (a) Are responsible for all phases of the operation of the hospital, selection of the medical staff, and quality of care rendered in the hospital. The review shall include the quality and necessity of the care provided and the preventability of complications and deaths occurring in the hospital. The records, data, and knowledge collected for or by individuals or committees assigned a review function described in this article are confidential and shall be used only for the purposes provided in this article, shall not be public records, and shall not be available for court subpoena. A hospital shall meet the minimum standards and rules authorized by this article and shall endeavor to carry out practices that will further protect the public health and safety, prevent the spread of disease, alleviate pain and disability, and prevent premature death. If consented to by the individual, the attending health care personnel shall perform or have performed on the individual the sexual assault medical forensic examination, including the procedures required by the sexual assault evidence kit. The hospital shall provide to the parents the information developed as required by subsection (2) on the purpose and completion of the form and on the rights and responsibilities of the parents. The hospital shall forward a completed acknowledgment of parentage to the state register for recording. The hospital shall provide assistance and training to hospital staff assigned responsibility for obtaining the forms, as appropriate. The acknowledgment of parentage form and information shall clearly state that completion of the form is voluntary on the part of the mother and father, and shall include all of the notices as provided in section 7 of the acknowledgment of parentage act. The hospital shall provide the information whether or not the hospital provides hospice care. This subsection does not apply if the hospital does not have electronic access to the information described in section 21541(1)(a)(i)(A) and (B). A hospital shall retain a copy of the notice required under this subdivision for not less than 7 years. You may be responsible for the costs of the transportation that is not covered by your health benefit plan. We have conducted a good-faith search to determine whether your health benefit plan provides coverage for this transportation and, if so, to order this transportation from a provider that participates with your health benefit plan. You have a right to be transported by a method other than transport by an aircraft transport vehicle or ambulance that is a rotary aircraft. The hospital and the ordering physician are immune from civil liability for injuries or damages arising out of your decision to use a form of transportation other than the one ordered by the ordering physician. A hospital shall not deny an aircraft transport vehicle or ambulance that is a rotary aircraft the right to land at the hospital for the purpose of allowing an aircraft transport vehicle that is a contracted provider with the hospital or ambulance that is a rotary aircraft that is a contracted provider with the hospital to remain on standby. The department shall grant an extension under this subsection unless the department determines under part 222 that there is a demonstrated need for the delicensed beds in the subarea in which the hospital is located. If the department does not grant an extension under this subsection, the hospital shall request relicensure of the beds pursuant to subsection (7) or allow the beds to become permanently delicensed pursuant to subsection (8). Upon receipt of a complete application under this subsection, the department shall temporarily delicense the beds indicated in the application. The department shall not grant an extension of temporary delicensure under this subsection. The form shall contain all of the following information: (a) the number and location of the specific beds to be delicensed. Along with the application, an applicant for delicensure under subsection (1) or (3) shall submit to the department plans that indicate to the satisfaction of the department that the space occupied by the beds proposed for temporary delicensure will be used for 1 or more of the following: (a) An alternative use that over the proposed period of temporary delicensure would defray the depreciation and interest costs that otherwise would be allocated to the space along with the operating expenses related to the alternative use. The department shall indicate in the bed inventory which beds are licensed and which beds are temporary delicensed under this section. The feasibility study shall include at least all of the following information: (a) the outstanding hospital bonded indebtedness and associated interest for all the hospitals in this state and the amounts payable in principal and interest per year until the bonds are retired. The committee shall be composed of 15 members equally divided among representatives of health consumers, health providers, and purchasers of health care. The advisory committee established under subsection (5) shall submit its report to the governor and the legislature not later than 4 months after the advisory committee receives the feasibility study. As used in this subsection, "medicaid" means that term as defined in section 22207. In developing recommendations under this subsection, the ad hoc advisory committee shall review the provisions of the code pertaining to hospital licensure in order to determine those provisions that should apply to rural community hospitals. The director shall direct the committee to report its recommendations to the department within 12 months after the committee is appointed. The director shall submit proposed rules, based on the recommendations of the committee, for public hearing not later than 6 months after receiving the report under section 21562(5). Except as otherwise expressly provided in this part or in rules promulgated under this section, a rural community hospital shall be licensed and regulated in the same manner as a hospital otherwise licensed under this article. The provisions of part 222 applicable to hospitals also apply to a rural community hospital and to a hospital designated by the department under federal law as an essential access community hospital or a rural primary care hospital. This part and the rules promulgated under this part do not preclude the establishment of differential reimbursement for rural community hospitals, essential access community hospitals, and rural primary care hospitals. The department may impose conditions upon a waiver under this section to protect the public health, safety, and welfare. A hospital that has entered into a contract with a community mental health board may establish a mental health crisis stabilization program for voluntary admission with a maximum length of stay not to exceed 72 hours. The center for rural health created under section 2612, as part of the development of the biennial rural health plan required under section 2223, shall develop a plan that provides for the creation of a set of rural health networks. Each rural health network shall consist, at a minimum, of 1 essential access community hospital, rural referral center, or regional referral center described in section 21566, and 1 rural primary care hospital as described in section 21567. Other rural health care providers including, but not limited to , primary care centers, community health centers, licensed nursing homes, and local public health departments may also be included in a rural health network for the purpose of developing a continuum of patient care. Population is to be determined according to the official 2000 federal decennial census. The purpose of a memorandum of agreement is to have a written understanding of the agreement between the parties. A memorandum of agreement serves as a legal document that is binding and holds the parties responsible to their commitment along with describing the terms and details of the cooperative agreement. A memorandum of agreement may be used between agencies, the public, the federal or state government, communities, and individuals. A permit application that is not timely filed is subject to a late fee in an amount determined by the department as the additional cost of processing the late renewal, but not more than a dental license late renewal fee. If the operator of the mobile dental facility changes, the permit is no longer valid. However, if an application for a new permit to continue operating the mobile dental facility is submitted not later than 30 days after the change of operator, the former permit is valid as an interim permit until the application is approved or denied, but not longer than 90 days. The memorandum of agreement shall state that the contracting dentist or party will accept referrals of patients treated at the mobile dental facility. The agreement to accept a referral does not require the dentist or party to treat the patient. A dentist licensed under this act need not be present at a mobile dental facility when only preventative dental services are being provided. If the patient is a minor or incapacitated person, the operator or his or her designee shall also attempt to contact a parent or guardian and inform him or her of the referral. Failure of the operator or his or her designee to comply with this subsection is cause for disciplinary action by the department. Upon request of the dentist or party who accepts the referral, the operator shall transmit all imagery records taken of the patient at the mobile dental facility. If the operator has a memorandum of agreement due to its status as a state of Michigan designated or funded oral health prevention program with oversight from the department, the operator is exempt from any requirement concerning a memorandum of agreement under this part. The residents, patients, personnel, or employees, other than food handlers, of the home are not required to submit to a medical or physical examination. However, the nursing home shall be inspected and licensed under laws pertaining to fire, safety, sanitation, and building construction. However, a nursing home may use the term "health center" or "health care center" or "rehabilitation center" or a term conveying a meaning substantially similar to those terms as long as those terms do not conflict with the terms prohibited by this subsection. The owner, operator, and governing body of a nursing home licensed under this article: (a) Are responsible for all phases of the operation of the nursing home and quality of care rendered in the home. A nursing home, regardless of its status as a legal entity, may employ or contract with an individual licensed or otherwise authorized to engage in a health profession under part 170 or 175 to provide the program of planned and continuing nursing care and medical treatment under this subsection, which care and treatment include direct clinical services to residents.
Purchase super cialis uk
Clinical Effectiveness and cost effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systemic review erectile dysfunction drugs recreational use cheap super cialis 80mg amex. Towards a public-private partnership for registries in the field of rare diseases. Drug Discov Today 14(23-24):1166-73 75 Yin W, Market incentives and pharmaceutical innovation. Is it time for a new evaluation system for payers in Europe to take account of new rare disease treatments Orphanet Journal of Rare Diseases 2012;7:74 81 McCabe C, Edlin R, Round J (2010) Economic considerations in the provision of treatments for rare diseases. Adv Exp Med Biol 686:211-22 82 Simoens S (2011) Pricing and reimbursement of orphan drugs: the need for more transparency. Orphanet J Rare Dis 6:42 83 Schey C, Milanova T, Hutchings A (2011) Estimating the budget impact of orphan medicines in Europe: 2010 2020. Last accessed Jan, 18 2013 114 Rare Diseases Orphan Product Development Act of 2002. S department of Health and Human services: Office of Rare diseases Research of the National center for advancing translational research Undiagnosed disease program rarediseases. S department of Health and Human services: Office of Rare diseases Research of the National center for advancing translational research Therapeutics for Rare and Neglected diseases. S department of Health and Human services: Office of Rare diseases Research of the National center for advancing translational research Bench to Bedside awards. A Referene Guide to the Food and Drug Administration Safety and Innovation Act, July 20, 212. Involvement of patient organisations in research and development of orphan drugs for rare diseases in europe. During this None; second product Ten years; can be reduced to product being approved for the extended from four to 10 period, no applications for with the same active six if orphan criteria no longer same indication unless clinical years registration and market ingredient will not be met superiority is shown approval of pharmaceuticals designated unless clinical of the same kind will be superiority is shown approved Other benefits Regulatory fee waivers, 50% tax Application fee reduced ( The year in the United States, more than to the National Association of Councils remaining minority of people with intel 10,000 cases of intellectual disabilities on Developmental Disabilities, 5. With early intervention, a functional replacement therapy, use of anti-Rh under the age of 18 are afected. At May Institute, and weaknesses in the areas of intellectual and adaptive behav we provide a wide variety of programs and services for individuals ior skills, psychological and emotional considerations, physical and families afected by developmental disabilities. Finally, a trained interdisci has programs in New England, the Southeast, and on the West plinary group of professionals should meet to determine what Coast that provide educational, residential, vocational, and day supports are needed to address each of the areas stated above. Cerebral Additional resources include: palsy is the second most common developmental disorder, fol American Association on Intellectual and lowed by autism spectrum disorders. Since its founding 55 years ago, May Institute has evolved into an award-winning national network that serves over 25,000 individuals and their families every year at more than 200 service locations in the Northeast, Southeast, and on the West Coast. The Institute operates several schools for children and adolescents with autism and other developmental disabilities. These care guidelines provide support and guidance to those practitioners caring for children during the initial 96 hours following a disaster. The Pediatric and Neonatal Care Guidelines are not meant to be all inclusive, should not replace an existing policy and procedure at a health care facility, nor substitute for clinical judgment. These guidelines are maintained by the Illinois Emergency Services for Children program and revised as needed. Rinse liberally with water, according to protocol, if suspected chemical exposure. There are sections within the following guidelines that provide guidance to providers in order to address their needs. Primary Assessment, Monitoring, Interventions and Key Points Assessment and Monitoring Interventions Key Points Airway Maintenance with Cervical Spine Airway Maintenance with Cervical Spine Motion Airway Maintenance with Cervical Spine Motion Motion Restriction Restriction Restriction Assess throat and nares. Position Stridor or noisy breath sounds indicate impending upper o Facial burns for optimal airway and suction as airway obstruction. Relative tachycardia is normal for burn need to perform more frequently if patient is unstable. Do not use fluid containing glucose for o Lund-Browder chart (See page 18 for printable version) fluid resuscitation. Ongoing Assessment, Monitoring, Interventions and Key Points Assessment and Monitoring Interventions Airway and Breathing Airway and Breathing Obtain chest X-ray if intubated, inhalation injury suspected or Supportive therapy and O2; wean as appropriate. Body Temperature Body Temperature Perform temperature checks based on health care facility nd rd With 2 and 3 degree burns, patients may have difficulty regulating their protocol. When supplies of blankets are depleted, patients can be wrapped in plastic wrap or aluminum foil for insulation and warmth. Splinting, Positioning and Mobility Splinting, Positioning and Mobility In a disaster, physical and occupational therapists may splint Obtain physical therapy /occupational therapy consult. Children are more vulnerable to maltreatment, abuse and abduction, if separated from their care giver. Community partners, such as the American Red Cross and National Center for Missing and Exploited Children, can assist with this process. The Patient Identification Tracking Form (Attachment 12 in Burn Surge Annex) should be utilized for all patients to assist with the reunification process. Psychosocial Address the psycho-social needs of burn patients o Immediate needs (pain, fear of unknown, similar to any trauma patient) o Long term needs (more ongoing, can need support for years) Treatment therapies may trigger traumatic response Explain any procedures. Palliative Care/Comfort Care During disasters, patients with extensive burn injuries may be triaged as Expectant based on the Burn Triage Guidelines. Some request the players patient being put on the floor; can be accommodated by lowering the bed all the way to the floor. Consult with hospital radiation safety procedures officer for assistance and identifying available instrumentation, if available. If child is unable to be evacuated to a safer area within 24 hours, contact Pediatric Care Medical Specialist for the possible need for repeat doses. Place one 130 mg tablet (or two 65 mg tablets) into a bowl and grind into a fine powder. Psychological Considerations Radiation emergencies, whether it be from a leak at a nuclear power plant or from a terrorist type incident such as a dirty bomb, leads to significant public anxiety. The anxiety associated with such events can appear out of proportion to the radiation induced health effects and can greatly affect the entire community. It is important for providers to determine if nausea is from contamination or from the anxiety of the event. Provide educational materials and counseling options to all patients and their families after a radiological emergency. Radioactive Contamination versus Exposure Radioactive contamination: radioactive material is on or inside a person o External contamination-radioactive material is only on outside of a person o Internal contamination-radioactive material is ingested, inhaled, or absorbed through the skin or open wound Radiation exposure: a person is exposed to radioactive materials Difference between contamination and exposure: o Person exposed to radiation may not be contaminated. Place a small piece of resuscitation or for feedings tape at that measurement to guide your placement depth. Some Tracheostomy Care (established parents will have a resource binder or other reference with them. If tube is accidentally dislodged and a replacement tube is not readily available, you may replace with an indwelling urinary catheter. This form is to be filled out by the initial hospital and sent with the patient (either when discharged home or to another facility) to communicate what initial management has been completed. Therefore, an evaluation of the newborn by a health care provider with expertise in the care of a newborn. The form below and information found in this care guideline is provided to assist those hospitals who typically do not care for newborns to provide necessary care until the above experts can evaluate the patient.
Purchase super cialis cheap
The boswellic acids have immunomodulatory effects P-glycoprotein and/or cytochrome P450 isoenzymes (see and are anti-inflammatory via a number of mechanisms erectile dysfunction doctors in ct cheap super cialis 80mg without a prescription. Pharmacokinetics, above), but the data are too sparse to make any meaningful predictions. Analysis of frankincense from various Boswellia species with inhibitory activity on human drug metabolising cytochrome P450 enzymes using liquid In an in vitro study, aqueous extracts of Boswellia serrata chromatography mass spectrometry after automated on-line extraction. However, the gum resin was found to have some Modulation of Pgp function by boswellic acids. Importance and management these data show that food intake can significantly increase the B Boswellia + Food bioavailability of boswellic acids, and suggest that Boswellia serrata extracts should be taken with meals, as therapeutic levels may not be achieved when taken on an empty stomach. Amoxicillin levels were still higher Bromelain + Tetracycline in the bromelain group 3hours after surgery. Bromelain appeared to the reason for this interaction is unclear, but it is possible that increase the serum levels of tetracycline by up to about fourfold. For information on the pharmacokinetics of individual flavonoids present in Constituents broom, see under flavonoids, page 186. Constituents Buchu leaf contains a volatile oil composed of diosphenol (buchu camphor), pulegone, isopulegone, 8-mercapto-p Interactions overview methan-3-one, menthone, isomenthone and others, and the An isolated case of lithium toxicity has been reported in a flavonoids diosmin, hesperidin, rutin and others. Use and indications For information on the interactions of individual flavonoids Buchu preparations are used as diuretics, for bladder and present in buchu, see under flavonoids, page 186. Caffeic, chlorogenic, ellagic and rosmarinic acids, and isopimarane diterpenoids are also present. Corticosterone secretion-inducing activity of saikosaponin metabolites formed in the alimentary tract. Anti-inflammatory and Bupleuri (root) 7 7 5 immune-modulatory activities have been demonstrated in Cinnamomi 1. Absorption of other derivatives, structural isomers and Liquorice, theirmonoglycosidesandaglycones,whichwereformedinthe page 272 gastrointestinal tract, depended on food intake. The pharma Hoelen 3 3 cological effects of saikosaponin a given orally may therefore Ostreae testa 2. Effects of Sho-saiko-to (Xiao-Cai-hu-Tang) on the pharmacokinetics of carbamazepine in rats. The diuretic effect of Sairei-to is the main constituent of a number of Chinese herbal mediated by nitric oxide production in pentobarbital-anesthetized rats. Sho-saiko-to slightly inhibits caffeine metab 3Ohnishi N, Nakasako S, Okada K, Umehara S, Takara K, Nagasawa K, olism. Neither sho-saiko-to nor sairei-to appears to alter the Yoshioka M, Kuroda K, Yokoyama T. Ohnishi N, Nakasako S, Okada K, Umehara S, Takara K, Nagasawa K, Yoshioka M, Sho-saiko-to slightly reduces the metabolism of caffeine, but this Kuroda K, Yokoyama T. Any modest effect is therefore absorption of saikosaponins (the main constituents of bupleurum) not directly attributable to bupleurum alone. Other main constituents of these one of 7 constituents, delayed and lowered (by 45%) the maximum products also seem unlikely to interact with ofloxacin. Although this tended to reduce the effect of Chinese medicines on bioavailability of ofloxacin in healthy volunteers. It was found that sho-saiko-to delayed gastric emptying, and so it could delay absorption of carbamazepine when given at the same Clinical evidence time. It is unlikely that sho-saiko-to or saiko-ka-ryukotsu-borei-to No interactions found. See maximum, sho-saiko-to increased the blood-glucose-lowering the table Constituents of some Chinese herbal medicines containing effects of tolbutamide by about 11%, which was not statistically bupleurum, page 89, for a list of the constituents. See the table Constituents of some second study found that sho-saiko-to decreases gastric emptying rate Chinese herbal medicines containing bupleurum, page 89, for a list (see also Bupleurum + Carbamazepine, page 90), which could of the constituents. Effects of Sho-saiko-to on the pharmacokinetics and pharmacodynamics of tolbutamide in rats. Burnet is used to treat infections, Not to be confused with Burnet saxifrage (Lesser burnet). Mechanism It is possible that the metal cations present in the extract may have formed chelates with ciprofloxacin thereby reducing its bioavail ability. Burnet was given in a clinically relevant dose, and the reduction in levels seen would therefore be expected to result in a clinically relevant reduction in the efficacy of ciprofloxacin. In general, ciprofloxacin should be taken at least 2hours before, and not less than 4 to 6hours after, the interaction between burnet and quinolones is based on drugs that it may chelate with, such as those containing polyvalent experimental evidence only. The majority of the quinolone antibacterials are known to interact with polyvalent Clinical evidence cations in the same way as ciprofloxacin, and it would therefore No interactions found. Influence of Sanguisorba officinalis, a mineral-rich plant drug, on the pharmacokinetics of ciprofloxacin in the rat. Not to be confused with Broom, page 85, which is Use and indications Cystisus scoparius (L. Blatterdock, Bog rhubarb, Bogshorns, Butterdock, Butterfly the pyrrolizidine alkaloids are hepatotoxic, and have been dock, Capdockin, Flapperdock, Umbrella plant. All parts of the plant contain sesquiterpenes and unsaturated pyrrolizidine alkaloids. During storage, some of the con Interactions overview stituents undergo transformation, thus the final composition Many theoretical interactions have been proposed, including of herbal preparations may vary depending on storage the suggestion that butterbur may interact through effects on conditions. Types, sources and related compounds Uses and administration Extracts of caffeine-containing herbs have been used Caffeine (1,3,7-trimethylxanthine, coffeinum, guaranine, medicinally for their stimulant and diuretic effects, and koffein, methyltheobromine, theine) is found in significant quantities, in approximate order of highest to lowest levels: may be promoted as slimming aids and for boosting energy. Caffeine may induce dependence, and stopping intake abruptly can cause withdrawal. It may also cause serious adverse effects 3 to 6 hours in adults, and is about twofold longer in people if used with other drugs or herbs with similar effects, such as who do not regularly consume caffeine. Caffeine may interfere with the dexamethasone of caffeine were similar whether consumed as coffee or suppression test, and the efficacy of adenosine and cola. Caffeine may raise clozapine levels, and has modest effects on the (a) Inhibitors of caffeine metabolism absorption of some analgesics, but probably does not There are a number of medicines that are known inhibitors of significantly affect lithium levels. A convenient five-drug lant and adverse effects of caffeine is seen in patients taking cocktail for the assessment of major drug metabolizing enzymes: a pilot study. Importance and management Importance and management An extensively studied interaction. Some consider that the modest increase in blood pressure of about 4/2mmHg with caffeine intake Caffeine can inhibit the effects of adenosine infusions used in has little relevance, whereas others consider it important. The manufac mind the possibility that caffeine intake from herbal supplements turers of adenosine state that xanthine-containing drinks (tea, coffee, might modestly increase blood pressure, and that this might not be chocolate, cola drinks, etc. Consider also Cocoa + Antihypertensives, page 140, Coffee 12hours of self-reported abstention from caffeine-containing prod + Antihypertensives, page 146, Cola + Antihypertensives, page 149, ucts. The authors suggest that a 12-hour abstention from caffeine should be taken more seriously. Blood containing products may be insufficient, and could result in false pressure response to chronic intake of coffee and caffeine: a meta-analysis of negative results. Evidence for an antagonism between caffeine and adenosine in the human cardiovascular system. Time for abstention from In a study in healthy subjects, caffeine citrate 120mg given with a caffeine before an adenosine myocardial perfusion scan. The effects of clonazepam, caffeine and the combination of the two drugs on human sleep. He had previously not had any problems when consuming caffeine coffee Experimental evidence while taking haloperidol 30mg and procyclidine 30mg daily.
Order discount super cialis on line
During expo home at any time of day or night and progress through a sures erectile dysfunction treatment comparison buy super cialis overnight, feared consequences and dysfunctional beliefs were self-paced workbook that allows the patient to design and discussed. Patients were followed for an addi nificantly higher proportion of patients (>50%) with min tional 14 weeks. The study is limited by small sample time of therapy, they had been taking the medication at a size, lack of double-blind ratings, and low doses of fluvox stable dose for at least 3 months. Practice Guideline for the Treatment of Patients With Obsessive-Compulsive Disorder 61 d. Patients in the wait-list condi patients on average had mild depressive symptoms. Both active treatments were superior to the helps patients maintain their gains (124). Nearly half the subjects were before any conclusion about the effectiveness of yoga can maintained on stable medication regimens, but this did be drawn. A direct comparison of the effects of clomipramine groups were instructed to practice at home daily. Post hoc analyses revealed that 1) both groups ramine plus antiexposure (n=12) in rituals and depression. At completed all 24 weeks (n=16, 13, and 15, respectively); of baseline, patients on average had mild depressive symp these 44, 19 (43%) entered with a major depressive or dys toms. The use of ad determine whether modifications in treatment regimens junctive antipsychotic medications and other promising can improve the proportion of responders and the degree, somatic treatments. For example, studies tion, deep brain stimulation) also need additional investi could show whether higher doses or more rapid titration gation. In designing such research, the treat knowledge of the underlying pathophysiologic basis of ment schedules investigated will likely differ with the goals the disorder and aid in identifying and developing new of treatment. Panic: How to Overcome Panic Attacks, Calm Phys ical Symptoms, and Reclaim Your Life. Bassett L: From Panic to Power: Proven Techniques Anxiety Disorders Association of America to Calm Your Anxieties, Conquer Your Fears, and Put You in Control of Your Life. Armstrong K, Best S, Domenci P: Courage After Fire: Coping Strategies for Returning Soldiers and Autism Society of America Their Families. New cation as Autism Therapists: Applied Behaviour Anal York, Barnes & Noble Books, 2004. Broadway United States, which are modeled on the 12-step program Suite 306 of Alcoholics Anonymous. National Council on Problem Gambling and Na 713-869-4902 tional Endowment for Financial Education: Personal A study of an intervention in which subjects are prospectively followed over time; there are treatment and control groups; subjects are randomly assigned to the two groups; both the subjects and the investigators are blind to the assignments. A prospective study in which an intervention is made and the results of that intervention are tracked longitudinally; study does not meet standards for a ran domized clinical trial. A study in which subjects are prospectively followed over time without any specific intervention. A study in which a group of patients is identified in the present and information about them is pursued retrospectively or backward in time. A qualitative review and discussion of previously published literature without a quantitative synthesis of the data. Nestadt G, Riddle M: Obsessive-compulsive personal sive disorder in a large health maintenance organiza ity disorder: personality or disorder Hollander E, Greenwald S, Neville D, Johnson J, with obsessive compulsive disorder. Angst J, Gamma A, Endrass J, Goodwin R, Ajdacic V, disorder among bulimic patients. Practice Guideline for the Treatment of Patients With Obsessive-Compulsive Disorder 77 41. Maina G, Albert U, Salvi V, Bogetto F: Weight gain clomipramine in treatment-resistant obsessive-compul during long-term treatment of obsessive-compulsive sive disorder. Practice Guideline for the Treatment of Patients With Obsessive-Compulsive Disorder 79 99. Wilhelm S, Steketee G: Cognitive Therapy of Obses fects of intensive versus twice-weekly sessions. New York, Guilford Press, 1993 [G] of inflated responsibility in the treatment of obsessive 144. Practice Guideline for the Treatment of Patients With Obsessive-Compulsive Disorder 81 155. Hohagen F, Winkelmann G, Rasche-Ruchle H, Hand serotonin reuptake inhibitors for obsessive-compulsive I, Konig A, Munchau N, Hiss H, Geiger-Kabisch C, disorder in children and adolescents. Szegedi A, Wetzel H, Leal M, Hartter S, Hiemke C: the American Psychiatric Association, 2nd ed. American Psychiatric Association: Practice guideline amine: a double-blind, placebo-controlled study.

