Viagra Plus
Generic 400 mg viagra plus overnight delivery
Table games physically beneficial for the human body erectile dysfunction protocol foods generic viagra plus 400mg online, modelled on outdoor sports. Curling stone; Shuffleboard; Similar sliding games 45 /00 Apparatus or methods for manufacturing balls 67/16. Tethered aerial top or spinner games (working of plastics or substances in a plastic state B29) 67/18. Badminton, shuttlecock, or like games with feathered missiles 47 /00 Devices for handling or treating balls 67/20. Boxing or football dummies 55 /00 Bags for golf clubs; Stands for golf clubs for use on 69/36. Stationarily-arranged devices for projecting balls propulsion features B60) (sling weapons F41B 3/00; traps for clay-pigeon 57 /00 Golf game accessories. Body-protectors for players or sportsmen (protective 65/00 Implements for throwing (throwing toys A63H 33/00; clothing or garments for sporting purposes throwing weapons F41B) A41D 13/00; in the form of caps or hats A42B 1/04; helmets A42B 3/00) 67 /00 Sporting games not provided for in groups A63B 1/00 to A63B 65/00 67/02. Disc roulettes; Dial roulettes; Teetotums; Dice-tops 3 /00 Board games; Raffle games (racing games, traffic games, or obstacle games characterised by figures 7 /00 Indoor games using small moving playing bodies, moved by action of the players A63F 9/14; aspects of. Chess; Similar board games game A63F 13/00; table games physically beneficial for 3/04. Lottos or bingo games; Systems, apparatus or devices A63B 67/00; miniature bowling games A63D 3/00; for checking such games [5] bagatelle or similar games A63D 13/00; billiards, pocket 3/08. Raffle games that can be played by a fairly large billiards A63D 15/00) [1,7] number of people 7/02. Shooting or hurling games (throwing-implements for A63F 9/00 covering the particular game. Dice (dice-tops A63F 5/04); Dice-boxes; Mechanical television screen, showing representations related to dice-throwing devices the game (electric circuitry, see the relevant subclasses 9/06. Patience; Other games for self-amusement (balls to therefor) [7] be shaken in small boxes A63F 7/00) 13/02. Racing games, traffic games, or obstacle games electrical digital computers G06F 3/00) [7] characterised by figures moved by action of the 13/08. Ferris wheels Swings; Rocking horses; Other toy animals for riding (as nursery furniture A47D 13/00rocking chairs as nursery furniture 29 /00 Rolling drums turning somersaults with or without A47D 13/00) [3] rolling seats 9/00 Swings Other apparatus for public amusement 11/00 See-saws 31/00 Amusement arrangements 13 /00 Cradle swings; Rocking-horses; Like devices resting 33 /00 Devices allowing competitions between several on the ground persons, not otherwise provided for 15 /00 Rocking horses on runners adapted for progressive movement 82 Int. Building blocks, strips, or similar building parts 13 /00 Toy figures with self-moving parts, with or without 33/22. Optical, colour, or shadow toys (kaleidoscopes movement of the toy as a whole G02B 27/08) 33/26. Magnetic or electric toys (electric drives 15 /00 Other gravity-operated toy figures A63H 29/00) 33/28. Soap-bubble toys; Smoke toys (blowing smoke rings Toy vehicles; Toy engines A24F 13/00) 17 /00 Toy vehicles. Imitations of apparatus, not otherwise provided for, (traffic games with figures moved by players. Government works Printed in the United States of America on acid-free paper 10 9 8 7 6 5 4 3 2 1 International Standard Book Number-10: 0-8493-9538-0 (Hardcover) International Standard Book Number-13: 978-0-8493-9538-3 (Hardcover) this book contains information obtained from authentic and highly regarded sources. Reasonable efforts have been made to publish reliable data and information, but the author and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. No part of this book may be reprinted, reproduced, transmitted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, microfilming, and recording, or in any informa tion storage or retrieval system, without written permission from the publishers. For permission to photocopy or use material electronically from this work, please access Trademark Notice: Product or corporate names may be trademarks or registered trademarks, and are used only for identification and explanation without intent to infringe. Preface We are honored to have been asked to carry on the tradition established by Dr. Polk in the first two editions of the Handbook of Biological Effects of Electromagnetic Fields. Their editions of this handbook were each recognized as the authoritative standards of their time for scientists working in bioelectromagnetics, the science of electromagnetic field effects on biological systems, and for others seeking information about this field of research. In revising and updating this edition of the Handbook of Biological Effects of Electromag netic Fields, we have expanded the coverage to include more material on diagnostic and therapeutic applications. At the same time, in updating and expanding the previous editions? coverage of the basic science and studies related to the possible biological effects of the electromagnetic fields, we have added new material on the related physics and chemistry as well as reviews of the recent developments in the setting standards for exposure limits. We asked the chapter authors to write what they would like to be the first thing they would ask a new graduate student in their laboratory to read. We hope that this edition, like its two predecessors, will be useful to many as a reference book and to others as a text for a graduate course that introduces bioelectromagnetics or some of its aspects. As a ?handbook? and not an encyclopedia, this work does not intend to cover all aspects of bioelectromagnetics. Nevertheless, taking into account the breadth of topics and growth of research in this field since the last edition, we have expanded the number of topics and the number of chapters. Unavoidably, some ideas are duplicated in chap ters, sometimes from different viewpoints that could be instructive to the reader; and different aspects of others are presented in different chapters. Because there is no sharp dividing line, some topics are dealt with in parts of both volumes. The reader should be parti cularly aware that various theoretical models, which are proposed for explaining how fields interact with biological systems at a biophysical level, are distributed among a number of chapters. No one model has become widely accepted, and it is quite possible that more than one will in fact be needed to explain all observed phenomena. Similarly, the chapters on bio logical effects of static magnetic fields and on endogenous electric fields in animals could equally well have been in the Biological and Medical volume. We have tried to use the index and cross-references in the chapters to direct the reader to the most relevant linkages, and we apologize for those we have missed. Bioelectromagnetics first emerged as a separate scientific subject because of interest in studying possible hazards from exposure to electromagnetic fields and setting exposure limits. A second interest is in the beneficial use of fields to advance health, both in diagnostics and in treatment, an interest that is as old as the discovery of electricity itself. Finally, the interactions between electromagnetic fields and biological systems raise some fundamental, unanswered scientific questions and may also lead to fields being used as tools to probe basic biology and biophysics. Answering basic bioelectromagnetic questions will not only lead to answers about potential electromagnetic hazards and to better beneficial applications, but they should also contribute significantly to our basic understanding of biological processes. Both strong fields and those on the order of the fields generated within biological systems may become tools to perturb the systems, either for experiments seeking to understand how the systems operate or simply to change the systems, such as by injecting a plasmid containing genes whose effects are to be investigated. Although any specific chapter in this work will empha size one or another of these threads, the reader should be aware that each aspect of the research is relevant to a greater or lesser extent to all three. The reader should note that the chapter authors have a wide variety of interests and backgrounds and have concentrated their work in areas ranging from safety standards and possible health effects of low-level fields to therapy through biology and medicine to the fundamental physics and chemistry underlying the biology. It is therefore not sur prising that they have different and sometimes conflicting points of view on the signifi cance of various results and their potential applications. Thus authors should only be held responsible for the viewpoints expressed in their chapters and not in others. We have tried to select the authors and topics so as to cover the scientific results to date that are likely to serve as a starting point for future work that will lead to the further development of the field. Some of the material, as well as various authors? viewpoints, are controversial, and their importance is likely to change as the field develops and our understanding of the underlying science improves. We hope that this volume will serve as a starting point for both students and practitioners to come up-to-date with the state of understanding of the various parts of the field as of late 2004 or mid-2005, when authors contributing to this volume finished their literature reviews. The editors would like to express their appreciation to all the authors for the extensive time and effort they have put into preparing this edition, and it is our wish that it will prove to be of value to the readers and lead to advancing our understanding of this challenging field. He was a Fulbright scholar in Baghdad, Iraq, in 1958 and joined the University of Colorado in 1959, where he is currently a distinguished professor. Gordon Prize from National Academy of Engineering for Innovations in Engineering Education 2004. Since 1992, he has been editor in chief of Bioelectromagnetics, an international peer reviewed scientific journal and the most cited specialized journal in this field. Between 1971 and 2000, he was part of an interdisciplinary research team investigating the biological effects of electromagnetic fields on biological cell cul tures.
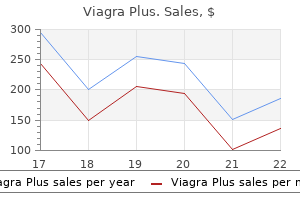
Buy 400mg viagra plus free shipping
Much as the total value of mergers and acquisitions fluctuated considerably from year to year erectile dysfunction unani medicine buy viagra plus from india, median disclosed transaction values generally increased between 2006 and 2015, with considerable fluctuation among years. Figure 7: Total Disclosed Value of Mergers and Acquisitions Conducted by Drug Companies, Overall, Largest 25, and All Others, 2006-2015 Notes: Totals do not include transactions with undisclosed values. Merger and acquisition transactions were attributed to the largest 25 if the company Bloomberg designated as the ?acquirer? in the transaction was one of the largest 25 drug companies by 2015 pharmaceutical and biotechnology sales revenue. Transactions attributed to all other companies included those for which Bloomberg designated a company other than one of the largest 25 as the ?acquirer. Substitutes can be products that are the same molecular entity or, in some cases, different molecular entities that treat the same condition. At levels narrower than the entire industry, such as drugs within the same therapeutic class or of the same molecular entity (levels that are more relevant to competition), concentration in shares of sales can be higher than in the overall industry. Seven of the 10 companies experienced small decreases in market share, while 2 companies?Novartis and Teva?experienced small increases (0. Greater numbers of generic manufacturers generally reduce concentration, as generic manufacturers compete with one another in addition to brand-name manufacturers. More broadly, one recent study found that of the novel drugs approved in tablet or capsule formulation since the 1984 Hatch-Waxman Act and eligible for generic competition, more than one-third had three or fewer generic approvals. EvaluatePharma estimated that in 2014, the 10 largest companies by prescription drug sales controlled about 44 percent of the overall pharmaceutical industry market, which still reflects lower concentration than in the therapeutic areas noted above. The latter study reported that the share of generic drugs (by dosage form, such as a tablet or injectable) with two or fewer manufacturers was relatively stable at about 50 percent between 2004 and 2016, which represents a relatively high concentration. Not all companies respond to in the Industry, Such as in those pressures in identical ways. For example, some experts said that the Types of Acquisitions some companies that traditionally manufactured brand-name drugs are and Increased expanding into the manufacturing of generic drugs. These brand-name companies may acquire a generics manufacturer to adjust the portfolio of Specialization in drugs they manufacture or gain access to a generics business. Similarly, Therapeutic Areas some traditionally generic manufacturers are expanding into brand-name manufacturing to acquire product lines with more generous profit margins. In addition, experts told us that investment in the development of traditional chemically synthesized drugs has produced increasingly lower financial returns, resulting in some traditional pharmaceutical companies turning to invest more in the development of more complicated and costly biologics. Many experts highlighted the proliferation of biotechnology companies as large pharmaceutical companies seek to acquire promising new research developments. Many experts told us that market pressures have also driven some drug companies to move towards specialization in certain therapeutic areas, including through mergers and acquisitions. Acquiring existing lines of business from competitors within a therapeutic area may also help a company increase its presence in a particular therapeutic area. Most recently, its merger attempt with Allergan was cancelled when, in 2016, the Treasury Department issued regulations designed to make it harder to invert. Claims for the Development orphan drug credit, one of several federal tax incentives encouraging drug development, increased sharply from 2005 through 2014. Biologics and Spending Grew orphan drugs accounted for an increasing share of new drug approvals Slightly, while from 2005 through 2016. Studies we reviewed and experts we interviewed suggested that potential revenues, costs, and policy Biologics and Orphan incentives influenced brand-name drug company R&D investment Drugs Were a Greater decisions. Estimates of worldwide R&D expenditures as a percentage share of total worldwide sales Slightly, from 2008 through averaged 13 percent and ranged from 11. Data represent pharmaceutical company-reported spending for R&D conducted in the United States regardless of the location of the parent company and spending for R&D conducted abroad by U. It does not include spending by biotechnology companies, which we reported separately because these estimates were less available and reliable. This included $44 billion on promoting drugs directly to providers?known as detailing?$8 billion for free samples, $8 billion for meetings, and $5 billion for direct-to-consumer advertising. Pharmaceutical companies include respondents with spending relevant to North American Industry Classification System code 3254 for pharmaceuticals and medicines; it does not include biotechnology companies. Data do not include industry spending for clinical trials conducted after the drug has come to market. For example, in 2014, self-reported R&D expenditures as a percentage of total sales were higher for pharmaceutical companies than for other comparably large, R&D-intensive sectors such as semiconductor and other electronic components, software publishers, and computer system design services. Specifically, R&D expenditure estimates were not available for 2008 or 2014 and estimates for years between varied greatly and with large standard errors. A more in-depth comparison of these industries was outside the scope of our review. In addition, state and local governments, foundations, charities, and venture capital also funded biomedical R&D, according to studies and experts we interviewed. Obligations represent amounts for orders placed, contracts awarded, services received, and similar transactions committed to by agencies during a given period, regardless of when funds were appropriated and when future payment of money is required. In 2015, National Health Expenditure estimates show that state and local governments spent $6. Pharmaceutical Company Pharmaceutical company spending from 2008 through 2014 focused on Reported Spending drug development, while federal spending focused on earlier-stage basic research. For example, in 2014 pharmaceutical companies reported Focused on Drug allocating 13 percent of total reported domestic R&D spending on basic Development and Federal research, 21 percent on applied research, and 66 percent on Spending Focused on development (see fig. Applied research includes activities aimed at solving a specific problem or meeting a specific commercial objective. Development includes systematic use of research and practical experience to produce new or significantly improved goods, services, or processes. In basic research the objective is to gain more complete knowledge or understanding of the fundamental aspects of phenomena and of observable facts, without specific applications toward processes or products in mind. In applied research the objective is to gain knowledge or understanding necessary for determining the means by which a recognized need may be met. Development is systematic use of the knowledge or understanding gained from research, directed toward the production of useful materials, devices, systems, or methods, including design and development of prototypes and processes. Pharmaceutical companies include respondents with spending relevant to North American Industry Classification System code 3254 for pharmaceuticals and medicines. Basic research includes activities aimed at acquiring new knowledge or understanding without specific immediate commercial applications or uses. Applied research includes activities aimed at solving a specific problem or meeting a specific commercial objective. Development includes systematic use of research and practical experience to produce new or significantly improved goods, services, or processes. Data do not include industry spending for clinical trials conducted after the drug has come to market. This includes supporting pre-clinical and early-stage clinical trials; promoting and initiating collaborations and partnerships among industry, academia, and other stakeholder communities, such as patient advocacy groups, to address research barriers; and facilitating data sharing, according to agency officials. Lichtenberg, ?What Are the Respective Roles of the Public and Private Sectors in Pharmaceutical Innovation? Toole, ?The Impact of Public Basic Research on Industrial Innovation: Evidence From the Pharmaceutical Industry,? Research Policy, vol. Avorn, ?The Roles of Academia, Rare Diseases, and Repurposing in the Development of the Most Transformative Drugs,? Health Affairs, vol. Data may overestimate federal spending directly applicable to drug research as it may include funds for biomedical research not directly relevant to drugs. Obligations represent amounts for orders placed, contracts awarded, services received, and similar transactions committed to by agencies during a given period, regardless of when funds were appropriated and when future payment of money is required. Basic research includes activities aimed at acquiring new knowledge or understanding without specific applications toward processes or products in mind. Applied research includes systematic study to gain knowledge or understanding necessary to determine the means by which a recognized and specific need may be met. Studies and experts we interviewed suggested that the relative roles of R&D funders and performers are evolving. For example, some experts noted that there is less distinction between public and private investment in R&D than in the past because publicly funded research institutions, such as universities, are frequently involved in financial relationships with industry for commercial development. Experts suggested that this trend is a response to the increasing complexity and cost of R&D concurrent with the advent of biotechnology and waves of patent and exclusivity expirations for large companies. Worldwide R&D spending paid for and performed by pharmaceutical companies decreased in real dollars from $61.
Diseases
- Tolosa Hunt syndrome
- Bone dysplasia lethal Holmgren type
- Premature atherosclerosis photomyoclonic epilepsy
- Plague, meningeal
- Peripheral nervous disorder
- Tay Sachs disease
Order viagra plus 400mg line
These cholesterol oxidation products can have major effects on cholesterol metabolism and have been shown to be highly atherogenic in animal models (Staprans et al impotence 23 year old order viagra plus with a visa. Overall, body cholesterol homeostasis is highly regulated by balancing intestinal absorption and endogenous synthesis with hepatic excretion of cholesterol and bile acids derived from hepatic cholesterol oxidation. As an example, many Tarahumara Indians of Mexico consume very low amounts of dietary cholesterol and have no reported developmental or health problems that could be attrib uted to this aspect of their diet (McMurry et al. The question of whether cholesterol in the infant diet plays some essential role on lipid and lipoprotein metabolism that is relevant to growth and development or to the atherosclerotic process in adults has been diffi cult to resolve. The idea that the early diet might have relevance to later lipid metabolism was first raised by Hahn and Koldovsky? This led to the hypothesis that cholesterol in human milk may play some important role in establishing regulation of cholesterol homeostasis. Since human milk typically provides about 100 to 200 mg/L (Table 9-1), whereas infant formulas contain very little cholesterol (10 to 30 mg/L) (Huisman et al. Formula-fed infants also have a higher rate of cholesterol synthesis (Bayley et al. Differences in cholesterol synthesis and plasma cholesterol concen tration are not sustained once complementary feeding is introduced (Darmady et al. Also, no clinically significant effects on growth and development due to these differences in plasma cholesterol concentration have been noted between breast-fed and formula-fed infants under 1 year of age. The effects of early cholesterol intake and weaning on cholesterol metabolism later in life have been studied in a number of different animal species (Hamosh, 1988; Kris-Etherton et al. Studies in baboons fed breast milk or formulas with or without cholesterol and with varying fat composi tions found that early cholesterol intake had little effect on serum choles terol concentrations in young adults up to about 8 years of age (Mott et al. These differences were not explained by variations in the saturated and unsaturated fat content of the formulas and milk. The major metabolic difference associated with the differences in plasma lipoproteins was lower rates of bile acid synthesis and excretion among the baboons that had been breast fed. The possible relations of early breast and bottle feeding with later cholesterol concentrations and other coronary heart disease risk factors were explored in several short-term studies and larger retrospective epide miological studies, but these observations are inconsistent (Fall et al. The disparate findings may be due to confounding factors such as duration of breast feeding, since human-milk feeding for less than 3 months was associated with higher serum cholesterol concentrations in men at 18 to 23 years of age, or the type of formula fed since formula composition, especially quality of fat, which has changed dramatically in the last century (Kolacek et al. The available data do not warrant a recommendation with respect to dietary cholesterol intake for infants who are not fed human milk. How ever, further research to identify possible mechanisms whereby early nutri tional experiences affect the atherosclerotic process in adults, as well as the sensitive periods in development when this may occur, would be valuable. High amounts of cholesterol are present in liver (375 mg/3 oz slice) and egg yolk (250 mg/ yolk). Although generally low in total fat, some seafood, including shrimp, lobster, and certain fish, contain moderately high amounts of cholesterol (60 to 100 g/half-cup serving). One cup of whole milk contains approxi mately 30 mg of cholesterol, whereas the cholesterol contained in 2 per cent and skim milk is 15 and 7 mg/cup, respectively. One tablespoon of butter contains approximately 12 mg of cholesterol, whereas margarine does not contain cholesterol. Dietary Intake Based on intake data from the Continuing Survey of Food Intakes by Individuals (1994?1996, 1998), the median cholesterol intake ranged from approximately 250 to 325 mg/d for men and 180 to 205 mg/d for women (Appendix Table E-15). The meta-analysis also identified a diminishing increment of serum cholesterol with increasing baseline dietary cholesterol intake. With a baseline cholesterol intake of 0, the estimated increases in serum total cholesterol concentration for intakes from 100 to 400 mg/d of added dietary cholesterol were 0. Other predictive formulas for the effect of 100 mg/d of added dietary cholesterol, which did not consider baseline cholesterol intake and are based on compilations of studies with a variety of experimental conditions, have yielded estimates of 0. Furthermore, pooled analyses of the effects of 100 mg/d of added dietary cholesterol on plasma lipoprotein cholesterol concentrations (Clarke et al. The incremental serum cholesterol response to a given amount of dietary cholesterol appears to diminish as baseline serum cholesterol intake increases (Hopkins, 1992). There is also evidence from a number of studies that increases in serum cholesterol concentration due to dietary choles terol are blunted by diets low in saturated fat, high in polyunsaturated fat, or both (Fielding et al. There is considerable evidence for interindividual variation in serum cholesterol response to dietary cholesterol, ranging from 0 to greater than 100 percent (Hopkins, 1992). There is increasing evidence that genetic factors underlie a substantial portion of interindividual variation in response to dietary cholesterol. An instructive case is that of the Tarahumara Indians, who in addition to consuming a diet low in cholesterol, have both low intestinal cholesterol absorption and increased transformation of cholesterol to bile acids (McMurry et al. However, with an increase in dietary cholesterol from 0 to 905 mg/d, their average plasma cholesterol concentration increased 0. Variations in several genes have been associated with altered respon siveness to dietary cholesterol. The common E4 polymorphism of the apoE gene has been associated with increased cholesterol absorption (Kesaniemi et al. The recent finding that apoE is of importance in regulating cholesterol absorption and bile acid formation in apoE knockout mice (Sehayek et al. There are numerous other candidate genes that could modulate plasma lipid and lipoprotein response to dietary cholesterol by affecting cholesterol absorption, cellular cholesterol homeostasis, and plasma lipo protein metabolism. Studies in animal models have generated data in support of the possibility that variations among these genes may be of importance in influencing dietary cholesterol response in humans, but to date such human data are lacking. Nevertheless, the existence of marked interindividual variability in dietary cholesterol response among and within various animal models points to the likelihood that some of the mecha nisms underlying this variability will also apply to humans. There is compelling evidence that dietary cholesterol can induce atherosclerosis in several animal species, including rabbits, pigs, nonhuman primates, and transgenic mice (Bocan, 1998; McNamara, 2000; Rudel, 1997). However, given the existence of marked inter and intraspecies differences in cholesterol metabolism and athero genic mechanisms, it is not possible to extrapolate these data directly to humans. A significant relative risk was also observed in the Western Electric Study, which remained significant after adjustment for a number of covariates, including dietary fat and serum cholesterol concentration (Stamler and Shekelle, 1988). More recently, in a study of 10,802 health conscious men and women in the United Kingdom, a univariate relation ship of cholesterol intake to ischemic heart disease mortality was observed (Mann et al. This finding was corroborated in a European study, but after multivariate analysis adjust ing for fiber intake, the association was no longer significant (Toeller et al. Measures of atherosclerosis using imaging techniques have also been assessed in relation to diet. Angiographically assessed coronary artery disease progression over 39 months in 50 men was weakly related to cholesterol intake in univariate, but not multivariate, analysis (Watts et al. In 13,148 male and female participants in the Atherosclerosis Risk in Commu nities Study, carotid artery wall thickness, an index of early atherosclerosis, was significantly related to dietary cholesterol intake by univariate analyses; multivariate analysis was not performed (Tell et al. Another uncertainty relates to interpreting the effects of dietary cholesterol on blood cholesterol concentrations. Finally, the considerable interindividual variation in lipid response to dietary cholesterol may result in differing outcomes in differ ent populations or population subgroups. Cancer As shown in Tables 9-5 through 9-8, no consistent significant associa tions have been established between dietary cholesterol intake and cancer, including lung, breast, colon, and prostate. Several case-control studies have suggested that a high consumption of cholesterol may be associated with an increased risk of lung cancer (Alavanja et al. As reviewed above, on average, an increase of 100 mg/d of dietary cholesterol is predicted to result in a 0. This effect of added cholesterol is highly variable among individuals and is considerably attenuated at higher baseline cholesterol intakes. Epidemiological studies have limited power to detect effects of such magnitude and thus do not provide a meaningful basis for establishing adverse effects of dietary cholesterol. However, no studies have examined the effects of very small increments of dietary cholesterol in numbers of subjects suffi ciently large enough to permit statistical treatment of the data. Because cholesterol is unavoidable in ordinary, nonvegan diets, eliminating choles terol in the diet would require significant changes in patterns of dietary intake. Independence of the effects of cholesterol and degree of saturation of the fat in the diet on serum cholesterol in man. Andersson S-O, Wolk A, Bergstrom R, Giovannucci E, Lindgren C, Baron J, Adami H-O. Energy, nutrient intake and prostate cancer risk: A population based case-control study in Sweden. Dietary fat and risk of coronary heart disease in men: Cohort follow up study in the United States. Influence of formula versus breast milk on cholesterol synthesis rates in four-month-old infants. Effect of egg yolk feeding on the concentration and composition of serum lipoproteins in man.
Buy viagra plus without prescription
Insurance companies should update their policies to be consistent with the most recent and relevant evidence-based medicine erectile dysfunction gene therapy effective 400 mg viagra plus, which expert consensus panels translate into management options with sound clinical reasoning. There were over 100 fixed-response questions that asked about 5 content areas: demographics, genetic testing, prophylaxis, childbearing, and partnering. Most (80%) women said that their health care provider offered them increased screening for the early detection of breast cancer. When the remaining 25 women were asked why they decided to not have surgery, 56% remained undecided, 40% said they were too young, 32% were delaying surgery to breastfeed, 24% wanted to avoid surgery, and 4% said their health care provider said it was not necessary. Those with 3 or more family members with breast cancer were no more likely to choose mastectomy (27%) than those with 2 or less (32%). Reasons for not having the surgery included indecision, being too young, delaying to breastfeed, and wanting to avoid surgery. Larger studies confirming our findings and exploring the perspectives of breast surgeons are needed. Data regarding compliance with subsequent recommendations is strikingly sparse in the literature. Herein, the aim is to assess the incidence of pathogenic mutations associated with breast cancer as well as compliance with screening and risk-reducing therapy recommendations within the context of an increased risk of breast cancer. Methods: A retrospective analysis of subjects evaluated due to a possible increased risk of breast cancer was conducted from January 2013-August 2016. Variables including genetic testing recommendations and results as well as compliance with recommendations for clinical follow-up, radiologic screening, prophylactic surgery, and risk-reducing medication were assessed. Fifty-eight percent (n=866) underwent genetic testing: 38% (n=79) were evaluated due to family history, 43% (n=89) due to personal history, and 19% (n=41) due to both family and personal history. Twenty-four percent (n=209) of those tested (14% of all subjects) were found to have a genetic mutation; 16% harbored pathogenic mutations associated with breast cancer. After excluding this group, 93% (n=126/136; denominator for analysis indicates those who received such a recommendation) complied with recommendations for clinical follow-up, 88% (n=88/100) complied with radiologic surveillance, 88% (n=52/59) complied with prophylactic mastectomy, 73% (n=52/71) complied with prophylactic oophorectomy, and 95% (n=41/43) complied with risk-reducing medication. Conclusions: the current report serves as one of the largest reports to date regarding compliance with recommendations within the context of increased risk of breast cancer. While nearly a third of subjects were lost to follow-up, those who did follow up demonstrated significant compliance with recommendations for screening and risk reduction. Given that a third of patients were lost to follow-up, further work is needed to identify barriers to compliance in this population, as well as insight into the outcomes associated with long-term compliance with such recommendations. It is less clear if patients who undergo prophylactic mastectomy are equally as affected as those with a cancer diagnosis. Responses were analyzed in total and divided into two subgroups: those with and without breast cancer. Among those without cancer, anxiety scores were not different between those choosing prophylactic mastectomy and high-risk screening. These programs were created to provide cancer risk assessment, genetic cancer screening, genetic cancer evaluation and testing, and development of a treatment plan with the patient in one location. Methods: It was determined the breast center would provide the screening questionnaire and Tyrer Cuzick score for each woman having a yearly mammogram or other breast exam. If the patient consented, testing could be performed the same day as consultation. If the patient had a positive genetic mutation, a referral was generated to the geneticist to develop a treatment plan. If the woman was high risk for breast cancer (Tyrer-Cuzick assessment of the lifetime risk of breast cancer to be greater than or equal to 20% or based on family history), she was referred to the cancer center to develop a high-risk breast cancer plan. During this third year, our population of hereditary cancer predisposition is at 7. Of those, 26 patients had a personal history of a breast, ovarian, prostate, pancreatic, or melanoma cancer. Conclusions: It is estimated 5-10% of all breast cancer can be attributed to a hereditary predisposition (National Institute for Health, 2017). A study in 2003 determined 9% of women with a significant family history warranted a genetic surveillance, lifestyle changes, medications, and/or surgeries to reduce their risk of cancer or ideally prevent cancer (Hughes, et al. During the past few years, our program has evolved from bringing genetic evaluation to a genetically underserved area, to developing a program that includes cancer risk assessment, genetic cancer screening, genetic cancer evaluation and testing, and developing a treatment plan all in one location. Most general surgery offices treat breast cancer, and in some cases, treat women at increased risk for breast cancer. General surgery needs to spearhead genetic testing in the breast cancer population. It is imperative to bring awareness for a genetics risk assessment to those who treat breast cancer the surgical office. This program was developed to reduce the risk of breast 68 cancer in our community. A program using a multidisciplinary approach should be utilized in general surgery and breast care clinics to perform genetic risk assessments. Clinical data and histopathology were analyzed from patient records, and 95% confidence intervals were calculated for proportions. All men presented with palpable masses, while approximately half of women had screen-detected breast cancer. Current practice guidelines for breast management in high-risk patients rely on personal/family history risk-based models, such as a Tyrer-Cuzick (T-C). Methods: For this retrospective analysis, 4,586 patients seen for a cancer genetics evaluation between September 2017 and September 2018 were queried from our internal database. Eighty-three percent of the population (n = 3,807) was eligible for T-C calculation. The mean age for patients with discrepant risk estimates (n=27, 26%) was 46 (range: 21-59). Cryoablation has the added advantage of being an image-guided percutaneous procedure that can be performed in the outpatient setting under local anesthesia. All patients in this trial underwent surgical resection to determine the success rate of cryoablation. Patients are treated with ultrasound guided cryoablation followed by 5 years of endocrine therapy. In this stratum, all patients will undergo Mammaprint testing on the core biopsy to determine risk of distant disease recurrence, and all will receive whole-breast radiation therapy post-ablation. Chemotherapy administration is left to the discretion of the treating medical oncologist. Patients are treated with ultrasound-guided cryoablation followed by 5 years of endocrine therapy. The secondary objectives are to determine ipsilateral breast tumor recurrence rate, axillary recurrence rate, breast cosmesis after cryoablation, and adverse events in patients treated with cryoablation alone. Results: Planned accrual is for 105 patients in each stratum with a total of 5 years of follow-up post ablation. Our institution has accrued 6 patients, 5 of whom have undergone ablation since May 2018. Two patients have reached 1 month of follow-up with no evidence of failure, recurrence, or adverse events. One patient has follow-up to 3 months without failure, recurrence, or adverse events. Conclusions: Cryoablation is a minimally invasive technique that can provide complete destruction of tumors, acceptable loco-regional control, good cosmesis, and minimal side effects in a selected population of women with early-stage hormone-positive breast cancer. As post-neoadjuvant lymph node status frequently influences surgical management, understanding the role of various imaging modalities for preoperative lymph node assessment is increasingly valuable to surgeons. We used sensitivity, specificity, and logistic regression to assess how well different modalities predicted final pathologic lymph node status. On final pathology, 24 lymph node-positive patients had measurable metastasis size with a median value was 0. In the absence of clinical concern for non response, there is little value for mid-treatment imaging due to its low specificity. They consisted of women with invasive lobular cancer, women with dense breast tissue, women with cancers that were difficult to see the primary, women with multiple apparent primaries, and young women under 50 years old who were diagnosed with breast cancer. If there were no recommendation, the primary care provider would simply refer to the surgeon. There were 30 patients evaluated prior to the initiation of this program and 31 patients evaluated afterwards. After we instituted routine advice given on the biopsy report from the radiologists, immediate incorporation of the recommendations was noted.
Cheap 400 mg viagra plus
In Asia impotence juicing discount 400mg viagra plus visa, government agencies in Japan, Malaysia, Saudi Arabia, Singapore, South Korea, Sri Lanka, and Taiwan have established regulatory pathways for the evaluation and approval of biosimilar agents. However, India has been producing ?intended copies? of already licensed biological products since 2007 under an abbreviated approval process that relies on limited efficacy data, thus allowing local biopharmaceutical manufacturers to keep production costs low and provide therapies to more patients than those who could afford the reference product. Figure 4 provides a timeline for the development of several global biosimilar agent approval pathways. Data source: Publicly available information from national regulatory authorities and World Health Organization guidelines and data on file. The guideline clearly states that biologics registered without a comprehensive, head-to-head comparison with a reference biologic should not be called ?similar biotherapeutic products. The guideline recommends a stepwise process for regulatory assessment of such products, taking into consideration the benefit-risk of keeping the products on the market, the missing elements from the original dossier, and an orderly procedure for obtaining additional required data from the sponsor. Biosimilars are biologics that are developed and approved according to the biosimilar regulatory pathway. A subsequent generic of a small molecule drug can be approved via an Abbreviated New Drug 43,54 Application (505[j] pathway) that shows the drug is an exact replica of the reference drug. Of note, clinical data generated from phase 3 pivotal studies evaluating a proposed biosimilar is not generally included on the label of the biosimilar product. Step 1: Extensive structural and functional characterization of both the biosimilar product and the reference product is the foundation for the biosimilar development program. This 18 analytical characterization includes the determination of differences in relevant critical attributes between a biosimilar and the reference product using appropriate methodology. If rigorous structural and functional comparisons show minimal or no differences between the proposed biosimilar product and the reference product, there is a stronger justification 5 for a more selective and targeted approach to animal and/or clinical testing. Nonclinical studies may not be warranted if a biosimilar has been demonstrated to be 5 highly similar to a reference product through analytical characterization. The goal of immunogenicity studies is to establish there are no clinically meaningful differences in incidence and severity of human immune response between the biosimilar and the reference product that can impact both safety and efficacy of the biosimilar. Immunogenicity studies should be conducted in a sensitive 5 population and include assessments of binding and neutralizing antibodies. To be granted an ?interchangeability? designation, a product must demonstrate that it can be expected to produce the same clinical result as the reference product in any given patient (Figure 6). The number and duration of switches between the reference product and the proposed interchangeable product should consider the clinical condition to be treated, the dosing of the product, and the duration of the exposure interval to each product that would be expected to cause the greatest concern in terms of immune response and the potential impact of such response on safety and efficacy, if any. The switching arm should incorporate at least two separate exposure periods to each of the two 20 products. It should be noted that a first biosimilar designated as ?interchangeable? with a reference product will be granted a period of exclusivity during which time another biological product may not be assigned as ?interchangeable? with that same reference product. Many state laws, however, also include provisions whereby a prescriber may prevent substitution by stating ?dispense as written? or ?brand medically necessary. Demonstration of biosimilarity is the first step, while demonstration of interchangeability has additional requirements. The use of unique cell lines and different manufacturing processes results in proteins that have unique structural characteristics compared to the original protein. These biophysical variations between biosimilar and reference formulations were observed in the absence of statistically significant variations in clinical parameters. Regulatory agencies around the world continue to emphasize the importance of clinical testing to evaluate the clinical impact, if any, of these minor biophysical variations. Practical Considerations for Biosimilars Biologics play an essential role in disease treatment and supportive care. Therefore, when biosimilar agents enter the market as potentially less-expensive biologic competitors, prescribers and other healthcare professionals will likely require more clinical data than typically utilized for 78 review of small molecule generic drugs in order to make informed decisions. In May 2013, the North American Center for Continuing Medical Education carried out a survey where over 400 healthcare professionals, including oncologists, pharmacists, rheumatologists, and primary care providers, were evaluated on their knowledge of biosimilars. An educational needs assessment of more than 200 practicing clinicians (including more than 50 pharmacists) in the United States indicated that although there is a significant interest in utilizing biosimilars in practice, there are clear knowledge gaps regarding the definition of biosimilars and their regulatory approval process. Almost all respondents (97%) indicated the need for more education related to biosimilars. Additionally, 37% of surveyed prescribers were unaware that clinical trials for a single indication led to approval for multiple indications. One of the most significant issues that will potentially hinder the adoption of biosimilars is a lack of information about these agents on the part of physicians, nurses, and other healthcare 83 professionals. Some key considerations related to the use of biosimilars in clinical practice are discussed below. In 2013, France passed a law permitting a restricted form of substitution, wherein pharmacists may dispense a biosimilar product for a patient who is initiating therapy and has been prescribed the 35 reference product. States vary on the terms of substitution for traditional chemical drugs, and it is anticipated that states will vary in how they apply substitution to 65 biologics. As of July 2017, 35 states and Puerto Rico have adopted laws regarding substitution of biologic medications by the pharmacist without the intervention of the prescribing physician, although the prescriber can prevent substitution by stating ?dispense as written? or ?brand medically necessary. Such communication should rely on prescriber-accessible electronic systems, if available, Substitution:*? a practice allowed or any other prevailing means of communication if such by law wherein a pharmacist may systems are not in place. No communication is necessary for dispense an alternative biologic refills where there is no change from the product originally medicine for a prescribed biologic medicine without the prior approval dispensed; and of the prescriber. In many ?Private organization management of countries, biologic medicines are specifically excluded from substitution may vary based on formulary decisions and other factors. These statements clearly differentiated this practice from pharmacy substitution, and they emphasized the need for the prescriber to be involved. This may be done based on the available knowledge of the reference product as well as the totality of evidence generated during development of the proposed biosimilar (Figure 8). Extrapolating indications does not, on its own, indicate that a biosimilar is interchangeable with its reference product. Concerns have been raised by various organizations about the efficacy and safety of biosimilars in extrapolated indications that have not been formally evaluated in clinical studies. Extrapolation of indications for the proposed biosimilar is based on the knowledge of the reference product and scientific justification. Due to their molecular size, biologics (both reference biologics and biosimilars) have the potential to stimulate unwanted immune reactions. Furthermore, because biologics are large, complex molecules and made in living cells, they are generally very sensitive to the manufacturing process, environmental conditions, container closure systems, and handling, and structural changes in the molecule can occur after the product has been approved. When biologics (reference biologics or biosimilars) cause unexpected or rare adverse reactions in patients, it is essential that these reactions be attributed to a specific product and manufacturer so that any problem with a product 18,31 can be promptly identified and addressed to ensure product efficacy and patient safety. In particular cases, such risks may need to be evaluated through postmarketing surveillance or studies. Post-marketing safety monitoring for a proposed biosimilar product should have adequate mechanisms in place to differentiate between the adverse events associated with the proposed product and those associated with the reference product. Some registries require the provider to record each administered dose of a product in a product-specific central database. Adherence to data-entry requirements is enforced by restricting distribution of the product to providers who have joined the registry. The major advantage of this model is that it maintains very complete data on exposures, and possibly outcomes, for as long as the registry is maintained. The major disadvantage is that these registries are very expensive to establish and maintain and are very burdensome for healthcare providers to use. Thus, 97 the utility of product-specific registries has been limited to very risky products. Lot numbers are also unique identifiers, but they are infrequently and inconsistently used. Associating adverse events with the correct product and manufacturer may become more challenging with the arrival of biosimilars, unless each biologic has a 78 distinguishable nonproprietary name. In the absence of distinguishable nonproprietary names, other significant policy measures would be necessary to facilitate product-level identification of all biologics in patient medical records and adverse event reporting. For example, the European law requires each biological product to be identified by a trade name and each member state to take measures to ensure that important identifiers are accurately recorded in patient medical records 96 and adverse event reports. For biosimilars, brand names are not required, and prescribers and other healthcare providers are not required to use them. The question has been raised about whether biosimilars should have unique nonproprietary names to ensure that they are not treated like multisource generic drugs for purposes of prescription ordering, health records, and pharmacovigilance (Figure 9). Furthermore, assigning the same nonproprietary name to all biosimilars of a given reference product could create challenges in prescribing and reimbursement if not all biosimilars are granted the same indications via extrapolation. It is important that health authorities, sponsors, healthcare professionals, and patients can rely on timely and accurate adverse event data to make critical decisions regarding the use of biologics. Distinguishable nonproprietary names and clear labeling may assist with traceability measures.
Cheap viagra plus online mastercard
However erectile dysfunction naturopathic treatment buy cheap viagra plus 400 mg on-line, in the health care market, the purchase of goods and services is largely influenced by health care providers, who may not be well informed about, or incentivized to consider, the prices involved. In the case of drugs, some experts argue that marketing and advertising may further distort provider decision making. In addition, if the patients? medical bills are largely paid by insurance plans (other than copayment or coinsurance costs), then patients? demand may not be significantly influenced by changes in price to the extent that it might be in other markets where the consumers see and pay the bill themselves. For example, Medicare Part D is required to cover all drugs in six protected classes, which some experts argue reduces the negotiating power of its contractors (known as plan sponsors). Some research and experts we interviewed have noted that this practice erodes the negotiating power of insurers and the cost management utility of formularies, which may result in lower prices for the patient using the coupon but higher prices overall. Part D sponsor formularies must include all or substantially all drugs in the following six classes of clinical concern: immunosuppressant (for prophylaxis of organ transplant rejection), antidepressant, antipsychotic, anticonvulsant, antiretroviral, and antineoplastic. Examples of other therapeutic classes include analgesics, blood glucose regulators, cardiovascular agents, dermatological agents, respiratory tract agents, and sedatives. Consolidation and the Experts have said that consolidation as a result of mergers and acquisitions is one of multiple factors that could influence competition. These agreements are subject to public notice and comment and result in an enforceable order. The goal of a merger remedy is to preserve or restore competition in the relevant 25A merger involves either the sale of all or part of the stock or assets of one company to another. In addition, private parties and states may enforce state and federal antitrust laws by bringing suit for violations of these laws. The number of mergers and acquisitions among companies in the industry generally held and Acquisition Deal steady from 2006 through 2015, but merger and acquisition deal values Values Increased, increased. Industry experts we interviewed noted that market pressures and the Industry have driven structural changes in the industry. The study considered a remedy for on-market drugs?those marketed by both merging companies?successful if the company to which the product was divested subsequently sold the product in the market. The study also examined divestiture relating to pipeline products?products in development by one or both of the merging parties?and considered these successful if all assets relating to those products were transferred to a new firm with the same ability and incentive to bring the pipeline product to market. Aggregate sales revenue for all other drug companies in our data grew more sharply, from $86 billion in 2006 to $206 billion in 2015?an increase of about 140 percent (see fig. Total sales revenue for these companies not limited to pharmaceutical and biotechnology revenue followed a similar trend. About 27 percent of pharmaceutical and biotechnology revenue in 2015 was held by the 5 largest companies. Drug companies? average profit margins also grew from 2006 to 2015, though the trends differed for the largest 25 companies compared to the remaining companies in our data. Profit margins were weighted by companies? reported pharmaceutical and biotechnology sales revenue for each year. Profit margins are weighted by drug companies? pharmaceutical and biotechnology sales revenue for each year. To better place large drug companies? profit margins into context, we conducted a similar examination of profit margins for large companies in other industries, specifically software companies and the largest 500 companies (by 2015 total worldwide revenue as reported in Bloomberg) 33As alternative measures of profitability, we additionally examined the average sales-weighted return on assets and the average sales-weighted return on equity. Trends were similar to the average sales-weighted profit margin trend presented here. Thirteen of the largest 25 pharmaceutical/biotechnology companies (by 2015 pharmaceutical and biotechnology revenue) would have otherwise been among the largest 500 companies (by 2015 total worldwide revenue), as would three of the largest 25 software companies. Although there may be similarities between certain drug and software industry trends, the circumstances behind those trends could differ. A more in-depth comparison of these industries was outside the scope of our review. Profit margins for software companies were weighted by their software sales revenue. Profit margins for the largest 500 companies were weighted by their total sales revenue, minus any pharmaceutical, biotechnology, or software sales revenue that may have been reported. Overall, the number of transactions generally held steady, with through 2015, but the 312 in 2006 and 302 transactions in 2015 (see fig. The number of Values Fluctuated mergers and acquisitions involving one of the largest 25 companies (by 2015 pharmaceutical and biotechnology revenue) increased from 29 transactions in 2006 to 61 transactions in 2015. Transactions include drug companies (including both pharmaceutical and biotechnology) merging with or acquiring an asset from a pharmaceutical or biotechnology company, potentially including the company itself. Transactions attributed to all other companies included those for which Bloomberg designated a company other than one of the largest 25 as the ?acquirer. For example, in 2009, there were three transactions each valued above $20 billion in real dollars, all of which were conducted by companies in the largest 25: Bloomberg officials told us that transactions involving private companies often have undisclosed values. In 2015, about half of the total merger and acquisition transaction value came from five transactions each valued over $10 billion in real dollars, including one very large transaction by Allergan for about $72 billion. Conversely, the share of the worldwide pharmaceutical R&D spending that was paid for by the company and performed by others, such as through purchased R&D services, increased from 25 percent in 2008 to 35 percent in 2014. Inflation-adjusted claims by all industries for Drug Credit Increasing the orphan drug credit increased five-fold between 2005 and 2014, from Sharply about $280 million to about $1. Specifically, nearly all claims were from pharmaceutical manufacturers, drug wholesalers, and scientific research and development corporations, including corporations that conduct biotechnology research and development. Claims for the other tax credit that incentivizes drug development?the research credit?were more stable than the orphan drug credit between 2005 and 2014. This may be due in part to the fact that we were not able to obtain a specific estimate for the research credits claimed by biotechnology companies. They do not include corporations that primarily conduct biotechnology research and development. These research spending differences can reflect both differences in the definitions of research spending in each data source and in the specific industry definitions used in the different data sources. The ability of companies to deduct research expenditures in the year they are incurred simplifies tax accounting for research spending and reduces the after-tax cost of research investments. The table also shows that the amounts shown as research expense on the financial statements of the same corporations were slightly higher than the amount deducted on tax returns in each year. Financial reporting can differ from tax reporting because (1) the underlying consolidated group of companies may differ, and (2) the rules defining items may differ between financial and tax accounting. For example, a new dosage or route of administration for an existing drug may improve patients? compliance with treatment. Biologics and orphan drugs each represented an increasing share of all drug approvals from 2005 through 2016. As shown in figure 15, biologics grew from 8 percent of all drug approvals in 2005 to 17 percent in 2016. Orphan-designated drugs as a share of all drug approvals grew even more dramatically from 5 percent of all drug approvals in 2005 to 21 percent in 2016 (see fig. Orphan drugs as a share of novel drug approvals ranged from 22 percent in 2007 to 42 percent in 2015. The Orphan Drug Designation program provides orphan status to drugs and biologics which are defined as those intended for the treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the United States, or that affect more than 200,000 persons, but for which the manufacturers are not expected to recover the development and marketing costs. The product categories that led the largest number of drug approvals fluctuated over time, but oncology drugs were among the most frequently approved in all but 2 years from 2005 through 2016. For the 22 novel drug approvals in 2016, the most common product categories were oncology (5 approvals) and neurology (4 approvals). Studies and Experts Studies and industry experts we interviewed, including economists and Suggest Potential industry association officials, suggested several drivers for drug company R&D investment decisions. These investment choices were influenced by Revenues, Costs, and revenue, cost, and regulatory and other policy incentives: Policy Incentives Influenced Drug Industry. Potential revenues: High revenue potential, typically associated with Research and a large potential number of patients or the potential for high drug prices, is an important incentive for R&D investment, according to Development Investment experts and some research. Studies show that potential market size, Decisions measured by revenue, is a determinant of R&D investment and market entry for both brand-name and generic drug companies. This includes investment in drugs for niche markets that may have limited competition, such as orphan drugs. Experts also noted that some companies invest to extend patent protection or exclusivity periods for existing drugs as a means to extend revenue generation by delaying or limiting the effect of generic competition? sometimes referred to as ?evergreening? or ?patent hopping. Myers, A Ricardian-Demand Explanation for Changing Pharmaceutical R&D Productivity, National Bureau of Economic Research working paper 22720 (2016). For example, exclusivity periods and patent protection, expedited review programs, and tax incentives were cited as influencing R&D investment.
LNA (Alpha-Linolenic Acid). Viagra Plus.
- Reducing the risk of heart disease and heart attacks.
- How does Alpha-linolenic Acid work?
- What is Alpha-linolenic Acid?
- High blood pressure.
- Are there safety concerns?
- Reducing the risk of pneumonia.
- Reducing the risk of hardening of the arteries (atherosclerosis).
- Dosing considerations for Alpha-linolenic Acid.
- What other names is Alpha-linolenic Acid known by?
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96991
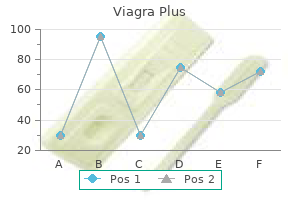
Purchase viagra plus 400mg overnight delivery
I found there to be a positive clockwise challenge of the hyoid bone (elevating the right side and lowering the left side of the hyoid simultaneously) erectile dysfunction evaluation order viagra plus 400 mg without prescription. Palpation and challenge were the primary tools for determining which muscles to address rather than the preset formula from the hyoid challenge. Palpation was for both muscles that felt they were either tighter or looser than they ought to feel. Treatment was applied according to the challenge, which in this case was opposite to the positive challenge since it was off of the spine and a non-rebound challenge. It should be noted that another distinction between my approach and the approach by Dr. In many cases I believe that the gentle application of pressure is just as if not more effective than using firm pressure. After her treatment the patient reported that she was able to breathe more easily, and she was shocked when she attempted to say ?Thank You. The second treatment included diversified and instrument assisted chiropractic manipulative therapy to the cervical and thoracolumbar spines. A positive challenge was found moving they hyoid laterally to the left hyoid and also in an inferior to superior direction. Results After the second visit her voice returned completely to normal by her standards, and her breathing and swallowing did the same. Palpatory tenderness and differences in the tension of the strap muscles and the other muscles in the neck were balanced. The patient did not return for follow up visits, but a mutual acquaintance reported that she returned permanently to her church choir which held a very high standard of performance. I have recollection of a similar case with a woman who was told that post thyroidectomy her voice could be hoarse for 6 months. After hearing this from her surgeon for many months and still experiencing dysphonia beyond that six-month mark she sought me out desperate to return to singing. As several authors are highlighting, the ?extralaryngeal skeleton? may be more of a cause in these cases rather than or possibly in conjunction with damage or palsy of the laryngeal nerves as is regularly blamed for vocal changes after thyroidectomy. One of the researchers notes that the strap muscles are commonly retracted substantially or even divided and re-sutured together during surgery. They further detail that suturing muscles back in place or together again is not always possible, especially in the case of the 1 sternohyoid muscle. As kinesiologists we know that all muscles large or small are necessary for optimal function, but this further outlines the great need to work with this population of patients. Beyond that it is the position of this author that kinesiologists hold a key that can uniquely offer an expedited recovery to thyroidectomy patients and should be investigated further as becoming part of the standard of care for these patients. Clearly larger scale studies are necessary, but this type of care could bring about more rapid recoveries and greatly decrease the negative effects of this surgery. Neri G, Castiello F, Vitullo F, De Rosa M, Ciammetti G, and Croce A, Post thyroidectomy dysphonia in patients with bilateral resection of the superior laryngeal nerve: a comparative spectrographic study. Dysphonia, Dyspnea, & Dysphagia Post Thyroidectomy Treated Mechanically Using Golgi Tendon Organ Challenge Nicholai Sorochinsky, D. It is for those who are approaching post term and also for decreasing pregnancy-related pain. Keywords Chiropractic, Pregnancy, Pelvic Pain, Golgi Tendon Organ, Inducing Labor Introduction It is well established that it is medically acceptable to deliver a baby at full term (38-42 weeks of pregnancy). However, it has been my personal and professional experience that more and more mothers report receiving pressure from their doctors or midwives to undergo chemical or mechanical induction treatments if beyond 40 weeks of pregnancy. When faced with this pressure from medical providers, mothers should be made aware of the alternative methods that can encourage a timely and healthy delivery. While centuries of traditions and anecdotes suggest that nipple stimulation, intercourse, long walks, castor oil, enemas, and the like, are effective ways to encourage labor, little science surrounds these options. While there is ample research on the topic on the timing of inducing labor, it is largely connected to the rupturing of membranes prior to the onset of labor. Peer reviewed literature is largely missing on the topic of alternative methods to safely and naturally encouraging the labor process to begin. While a handful of articles include the alternative yet frequently used methods, I was unable to find specifics on just how effective or ineffective any of them actually are, let alone with the backing of any statistical analysis. Clearly, the medical research will have a bias toward using medical methods, but in this case report I hope to outline a couple simple things to the reader. Third, even very late in the pregnancy these methods can be very helpful for quickly decreasing or even eliminating pregnancy related pain in the low back and pelvis. Discussion A slight-framed 29-year-old female presented to the office at 41 weeks pregnant with her first child. She had a healthy pregnancy thus far but was very concerned about the artificial induction process that her doctors were recommending. She had shown no signs of labor starting and had tried the traditional methods of long walks, warm baths, intercourse, etc. Recently, she started to note moderate groin pain bilaterally, lumbosacral junction pain, and left or right sided hip pain depending on the side she was sleeping on. As her pregnancy progressed and her breasts increased in size she noted an increase in mid-backpain which typically only bothered her later in the day. Since the patient was too uncomfortable and exhausted to do many manual muscle tests most of my treatment relied on palpation and challenge to direct where treatment was applied. The left pubis was palpated and challenged to be inferior, the sacrum to be posterior and superior on the right, L2/3 and L5/S1 to be counter rotated. The left gluteus medius and maximus were palpated and found to be mildly tender as were the bilateral rectus abdominis and pyramidalis muscles. All of these muscles were palpated and while not significantly painful or tight, they did stand out in relationship to the surrounding muscles. In other words, for all the muscles that left the axial skeleton and attached to the extremities the challenge was not a rebound. Corrective pressure was applied in the direction opposite of that which caused the strong indicator muscle to become inhibited. This particular muscle test is ideal for those who are in pain or who have low energy and do not have the stamina to repeat longer lever manual muscle testing. For these manipulations I used the Erchonia Adjustor set to multiple pulses per second with the pressure knob dialed back to make the impact less on the tender points of contact. The left round ligament was also treated in a Webster-like style in accordance to the right posterior and superior listing of the sacrum. While she was grateful for the relief she received she was most grateful for what happened immediately after her treatment. Before she made it to her car she felt the baby drop significantly lower in the pelvis and shortly after making it home she began active labor. The patient did not need to use induction to start labor and was able to successfully deliver her daughter naturally. It also gives us the ability to apply effective treatment with minimal pressure to the patient while taking the spinal correction to the next level by improving joint motion and stability by correcting the underlying muscle dysfunction. In another similar scenario a mother with severe central pubic pain presented to my office at 39? She had been dealing with this pain for several weeks, and it had been increasing to the point where she could no longer handle the pain. Her pain also instantly resolved in one treatment, and her labor began two days post treatment. Corneal in his office he stressed to me that palpation skills were probably the most important in determining where to apply this therapy. For doctors who rely heavily on their palpation or for those situations when palpation is practically the only option for detecting the need for treatment, this technique in my perspective is highly effective. It should be noted that manipulation of the muscle spindle cells is also an option. They respond in similar fashion to challenge and can be useful in avoiding contact in personal space especially about the pelvic attachments. Furthermore, it should be noted that these treatments are great options for those needing gentle care. This comes in very handy when dealing with populations that cannot tolerate heavy pressure, who are in acute pain, or who have other trauma that contraindicates the use of deeper pressure as in fracture, skin lesions etc. Even late in the pregnancy these options also offer the relief 31 of structural pain in relating areas.
Discount 400mg viagra plus overnight delivery
Note: various characteristics of swimming tend to promote urine production most aquatic athletes know of the urge to visit the bathroom during a workout erectile dysfunction tumblr buy generic viagra plus. If this occurs, you should try to estimate the volume of urine lost either, weigh yourself before and after the bathroom visit, or in the case of a research protocol where extra accuracy is required, collect the urine in a container so that you can measure the volume. This volume/weight should be subtracted from the pre-post session weight change to correct it to reflect sweat losses more accurately. To convert sweat loss over the session to a sweat rate per hour, divide by the exercise time in minutes and multiply by 60. Example: sweat loss of 2500 ml in 100 min session = 2500/100 x 60 = 1500 ml/hour 6. Your weight deficit at the end of the session provides a guide to how well you hydrated during the session, and how much you need to rehydrate afterwards. To convert kg to % body weight, divide the weight deficit by starting body weight and multiply by 100: Example: 1. Furthermore, o allowing more consistent and intensive training by the athlete should recognise that the sports food market increasing vigour during a workout or promoting includes products that are carefully manufactured to recovery between sessions provide nutrients to meet well documented goals right through to gimmicky items that have a poor composition o promoting adaptations to training or the addition of ingredients with no evidence base. Athletes should look carefully at o addressing a nutrient deficiency the risks and rewards of individual supplements before trying them. This is most vitamin or mineral, and an appropriate intake from food often the case just prior to , during, or after an is not possible, a supplement may be helpful. The use of supplements, however, does not discipline, the performance level, the training status and compensate for poor food choices and an inadequate also on the biochemistry and physiology of the individual. A much better option is to develop the knowledge and skills to ensure that all your nutritional needs are met Sports foods are generally manufactured to achieve the from a variety of well-chosen foods. Protein powders and supplements Examples of sports foods that might be useful include: Protein supplements, high protein bars and amino acid o sports drinks (providing fluid and carbohydrate preparations are among the biggest selling sports during exercise) nutrition products. In addition, they can provide an easily There is some evidence that athletes who are training consumed source of energy in a high energy diet hard may be at increased risk of minor illnesses and or while travelling infections. In themselves, these are generally trivial, but they can interrupt training or cause an athlete to miss o A simple whey protein powder. This may be useful when repair and adaptation is the main recovery need, or when a Many nutrition supplements, including glutamine, zinc, quick fix is needed to add quality protein to a sub echinacea, colostrum and others, are on sale with claims standard meal. There is no evidence that fancy that they can boost the immune system, but there is no versions of whey protein with special preparation strong evidence that any of these is effective. A strategies to support a healthy immune system include serve providing 20-30 g of whey protein is scheduling appropriate rest periods, and matching adequate to meet needs at a single meal or snack carbohydrate intake to fuel needs. There is good evidence that carbohydrate intake during Fat reduction and muscle building prolonged exercise reduces the release of stress hormones. There is also emerging evidence that A huge array of supplements is on sale with claims that probiotics, such as the lactobacillus found in dairy they can reduce body fat levels and build bigger and products, may also assist gut health and the immune stronger muscles claims that appeal to athletes and system. Many weight-loss supplements have been shown to contain prohibited drugs that are not listed on the label, principally from the stimulant category. Many of the muscle building products contain banned prohormones and compounds related to testosterone or anabolic steroids. Increasing energy supply and increasing training capacity Pre-workout supplements or pre-trainers are an Supplements for bone and joint health increasingly popular group of products which typically contain a large number of ingredients claimed to provide Hard training puts extra wear and tear on the bones, or enhance muscle fuel or increase vigour and capacity joints and associated structures, and numerous for training. The ingredients include substances like supplements are claimed to look after these tissues. In most cases these nutrients can be supplied In fact, in many cases, the ingredient list of these by a well-chosen diet and appropriate sunshine exposure. None of these is likely to improve glucosamine treatment can provide subjective relief in performance and, in spite of advertising claims, none is elderly individuals suffering from osteoarthritis, but supported by good independent evidence. There is now evidence is lacking for a benefits such as a ?joint limited evidence that carnitine can affect exercise protective? effect from high-intensity training in healthy athletes. Supplements that might work While energy drinks may seem refreshing and envigorating, they may be potentially dangerous if used There is scientific evidence to support the prospect of in excess or in combination with other stimulants or improved performance following the use of some alcohol. Importantly, they may be tainted with prohibited supplements in specific events, according to tried and substances, such as those derived from unregulated tested protocols. Buffering agents Creatine During very hard exercise, the muscles produce lactate Supplementation with creatine monohydrate can and hydrogen ions (acidity). This is both good (giving increase the amount of high energy phosphocreatine energy to allow hard efforts) and bad (causing pain and stored in the muscles, and may improve performance in interfering with muscle function). It may also lead to a gain in excess stomach acidity can be neutralised by taking strength and/or muscle mass, which is helpful for some bicarbonate, so can taking sodium bicarbonate (in a dose athletes but the extra weight may be harmful for others. This can reduce the fatigue and in meat and fish, but the effective doses (10-20 g per day performance decline seen in all-out events lasting from for 4-5 days to load, and then 2-3 g per day for about 30 seconds to 8 minutes, such as middle distance maintenance) are more than is found in normal foods. There is some evidence that this might improve performance in some high intensity exercise models, but further work is required to be sure of the range of situations in which it might be useful. Nitrate In untrained and moderately fit individuals, a few days of supplementation of the diet with nitrate has been shown A small amount of caffeine (1-3 mg/kg) can help to reduce the amount of oxygen required to do a set performance in prolonged exercise and may also be amount of work. For example, 100 mg of caffeine is supplied by a small More research is needed to confirm the efficacy of cup of brewed coffee or 750 ml of a cola drink. Larger beetroot juice/nitrate supplementation in well-trained doses of caffeine do not seem to be more effective, and aquatic athletes and to determine the events in which it may have negative outcomes such as anxiety, might be useful. This is likely to be a problem in additional nitrate in the form of vegetable intake, the multi-day events and in sports involving heats and finals. Note that energy drinks drinks containing sugar and caffeine should not be confused with sport drinks which 24 Nutrition for Aquatic Athletes Supplements and doping Athletes who are liable for drug testing under national or international programs should be especially cautious about supplement use. Some supplements are prepared in unhygienic conditions and contain substance that can cause health issues. Others do not contain some or all of the ingredients especially the expensive ones that are listed on the label. Contamination of dietary supplements with substances that may cause an athlete to record an Anti-Doping Rule Violation is widespread some surveys have suggested that as many as one in four supplements may result in a positive urine test. These prohibited compounds have not been declared on the label, so there is no way for the athlete to know that they are present. Purchases through the internet pose an even greater risk, and extreme caution should be taken. The only way to be sure is to avoid supplements altogether, but many athletes are unwilling to accept this advice. The sensible athlete will want to see very good reasons for using a supplement and a very low risk of an adverse test before deciding to use it. Many herbal supplements are claimed to increase testosterone levels and hence have an anabolic action. These include: Tribulis Terrestris; Chrysin; Indole-3-Carbinol; Saw Palmetto; Gamma-oryzanol; Yohimbine; Smilax; Mummio. All of these claims are based on studies in test tubes and none has been shown to work in humans. Athletes must be aware of the strict liability principle that makes them responsible for everything they eat and drink. Many international athletes, including some in aquatic disciplines, provide case studies of the misfortune associated with poor decisions regarding supplement use. Many have had to serve bans, prohibiting their participation in their sport for up to two years, due to an Anti-Doping Rule Violation associated with the use of a contaminated product. Check all supplements with a medical officer or qualified sports nutrition professional. Issues to consider when deciding to use a sports food or supplement 25 Nutrition for Aquatic Athletes Changing Body Composition Gaining Muscle Losing Fat Body mass and composition, including lean mass and fat focus on changes between assessments (?your mass, contribute to the success of athletic performance muscle mass has increased since the last in most aquatic events. Unfortunately, many athletes and measurement?) their coaches have expectations that there is a single o Results should be used to help explain changes in ideal physique for an event that can be achieved on performance outcomes, and to recommend or demand. In fact, optimal physique is individual to each support changes in diet or training strategies athlete, and is the result of genetics and many years of conditioning via good eating and specialised training. Even at the elite level, most aquatic athletes periodise Athletes often have a desire to change their body their body composition over the year and may only need composition to either reduce body fat stores or to to be in top shape for the most important competitions. This can be done by A regular program of body composition assessment can either altering energy intake, altering energy expenditure assess whether an athlete is making progress towards or both components. In all cases where energy balance is short term and long-term physique goals that will benefit changed to produce a loss of body mass and fat mass, performance as well as maintain health. If such protocols are used, they should be while sustaining lean body mass: undertaken by well-trained people who can achieve reliable measurements and provide suitable feedback. It o Set realistic goals and timeline for fat/weight loss, is important that such assessments are made in a preferably in the off-season professional, non-threatening environment with the information being provided to athletes in a meaningful o Meet with a sports nutrition expert such as sports way: dietitian for an assessment and an individualized plan o Athletes should be informed that the purpose of the assessment is to: o Try to create small energy deficits (no more than -400 kcal at any time during the day) by adjusting? Monitor any potential unhealthy changes that meal frequency and volume relative to energy may occur expenditureAvoid skipping meals, since this can?
Buy viagra plus 400mg with amex
The repeated attempts of lines erectile dysfunction over 75 400 mg viagra plus overnight delivery, these patients include those who have sustained placement and using more advanced modalities such severe blunt and penetrating torso and abdominal in as fluoroscopy to determine placement can increase juries, severe head injuries, major burns, undergone costs of providing care. Meta-analysis of clinical out 20 A Guide to the Nutritional Assessment and Treatment of the Critically Ill Patient 2013 Nutritional Support comes of several small sample size studies have evalu-. Use of bowel motility agents such as ated mortality, incidence of pneumonia, and reducing metoclopramide aspiration risk. It also prevents passage of bacte creased risk of reflux, aspiration, and pneumonia. A minimum daily tion, the following practices have been proven to reduce amount of 100?150g/day is necessary to provide ade the risk of aspiration:10,84 quate glucose to the brain. Higher percentages of mass and contractility protein may be needed in patients with ?wasting syn-. Increased bacterial colonization of energy needs have been associated with fever, im-. Emphysematous changes to lung paired immune function, liver dysfunction, and hy parenchyma105,106 potension. Be mended optimal level of intake for vitamins, minerals, tween 30?60% of inpatients and 10?45% of outpatients and electrolytes. Fluid requirements are estimated at 1 ml/kcal/day or Malnutrition may be responsible for the respiratory 20?40 ml/kg/day. Similarly, long-term caloric malnutrition is associ fecal, blood, wound, emesis) and with excessive insen ated with the loss of body weight that includes an sible losses (fever). Stress Response in 25 Critical Illness 0 10 20 30 40 50 Days After Injury Used with permission. Nutritional support is also an impor sion, poverty, difficulty shopping, and tiring easily when tant therapy in critical illness as it attenuates the meta preparing food often prevent good nutrition. Omega-3 fatty acids are metabolized to sub stances that reduce inflammation and inflammatory Stress Response in Critical Illness mediator production. Several studies observed reduced phases: the stress phase, the catabolic phase, and the duration of mechanical ventilation, number of days in anabolic phase. Hy Omega-6 fatty acids are metabolized to proinflam pometabolism and insulin resistance is also seen. The matory substances that influence cytokine production, primary goal during this time period is resuscitation platelet aggregation, vasodilation, and vascular perme and metabolic support. In hyperca orders such as coronary heart disease, diabetes, arthri tabolism, increased oxygen demands, cardiac output, tis, cancer, osteoporosis, rheumatoid arthritis, and and carbon dioxide production are seen. Caloric needs may be increased 24 A Guide to the Nutritional Assessment and Treatment of the Critically Ill Patient 2013 Nutritional Support Table 7. Consequences of Over Underfeeding Overfeeding Underfeeding Physiologic stress Increased complications Respiratory compromise Immune suppression Prolonged mechanical ventilation Prolonged hospitalization Hyperosmolar state Respiratory compromise Hyperglycemia Poor wound healing Hepatic dysfunction Nasocomial infection Excessive cost Prolonged mechanical ventilation Immune suppression Fluid overload Axotemia Used with permission. Underfeeding can result in a loss of lean body verse effect of hyperglycemia in patient outcomes. Hy an inability to respond to hypoxemia and hypercapnia, perglycemia is a normal response to physiologic stress and a diminished weaning capacity. Since hyperglycemia can be caused by enteral and deficit, which increases length of stay, days of mechan parenteral nutrition, control of hyperglycemia during ical ventilation, and mortality. The stress Overfeeding patients can be equally detrimental as response to critical illness causes wide swings in nutri well. Requirements for micronutrients are lean body mass while encouraging the use of adipose A Guide to the Nutritional Assessment and Treatment of the Critically Ill Patient 2013 25 Nutritional Support tissue for fuel. Morbidly obese patients receiving high trition are more complicated than those for adult pa protein through permissive underfeeding have reduced tients. Requirements for vita missive underfeeding where total calories are reduced mins and minerals vary based on age, medical status, while compensating with increased protein intake may and size of the child. Both of these conditions can be responsive to immunonutri Pediatric Critical Illness tion therapy. Immunonutrition using immune modu Optimizing nutritional therapy in pediatric patients lating nutrition formulations containing omega-3 fatty can improve clinical outcomes. As in the adult, the acids, arginine, glutamine, nucleotides, and anti goals of pediatric nutrition support encompass preser oxidants are used with the goal to modulate the im vation of tissue stores and resolution of disease mune system, promote wound healing, attenuate the progress. A recent multicenter international study inflammatory response, and improve organ function. A calorie is a unit of energy activity during a post-absorptive period 12?14 hours equivalent to the amount of potential heat produced after the last meal. A calorie (also called used in clinical practice to predict or measure caloric a large calorie, abbreviated as Cal) is defined as the needs. Kilocalo additional factors such as temperature, body surface ries (kcal) are used to quantify the energy value of area, diagnosis, and ventilation parameters, as shown foods. The received, and obesity) and have been added to the re calorie contribution of the three major macronutrients gression correlation equations. Several predictive equa are: protein = 4 kcal/g; carbohydrate = 4 kcal/g; and fat tions were developed with a focus on specific patient = 9 kcal/g. Predictive equations have varying degrees of agree Energy needs vary according to activity level and ment compared to measured calorie requirements. Energy needs of critically ill patients can Error rates are not insignificant and, therefore, can be significantly different than normal values. Addi have high degrees of under and overestimation of tional factors such as energy expended in catabolic caloric needs. This variability can result in errors large states may be needed to adjust the estimate in patients enough to impact outcomes. For ries and their correlation to height, weight, age, and example, in obese patients, there are no clinical fea gender in normal subjects to estimate the basal meta A Guide to the Nutritional Assessment and Treatment of the Critically Ill Patient 2013 27 Determining Nutritional Requirements Table 8. Many were developed as long as 50?80 tritional risk assessment process and sensible clinical years ago and may not reflect body composition, nu judgment when deciding whether to use a predictive tritional risks, age, or ethnicity of the populations they equation. The American Col are large segments of populations in whom predictive lege of Chest Physicians? 1997 equation is a simple and equations have no validation studies preformed. This 28 A Guide to the Nutritional Assessment and Treatment of the Critically Ill Patient 2013 Determining Nutritional Requirements Is the patient critically ill? Calculations of calorie re tor listed as follows:167 quirements by mathematical equation were developed from the use of direct and indirect calorimetry. Goal Energy Needs (kcal/kg) Weight maintenance 25 to 30 Direct calorimetry Weight gain 30 to 35 Direct calorimeters measure heat. A bomb calorime Weight loss 20 to 25 ter measures the energy value of food by measuring the To overcome the limitations of predictive equations precise amount of heat liberated as the food is burned and estimating formulas, energy needs can be meas in a closed chamber. Caloric Equivalence Substrate Respiratory Oxygen Carbon Dioxide Quotient Caloric Equivalent Caloric Equivalent (kcal/L) (kcal/L) Carbohydrate 1. However, the accuracy of this assumption has are measured at the airway, which simplify the meas never been substantiated. Bias flow through the ventilator may affect also been integrated into several ventilators. A Guide to the Nutritional Assessment and Treatment of the Critically Ill Patient 2013 31 Determining Nutritional Requirements Figure 13. MedGem handheld indirect calorimeter measures oxygen consumption to calculate resting energy expenditure using the caloric equivalent of oxygen. Measurements Resting energy expenditure from indirect calorimetry Used with permission. Current recommendations are to decrease the reliance on predictive equations in critically ill patients. Impact of Energy Balance on Clinical Outcome Impact of energy balance on clinical outcome (*p < 0. The ered for incorporation into a standard nutrition assess accuracy of equations 6, 7, and 9 above compared to the ment and treatment process. Additional validation Weir equation are within clinically acceptable limits studies and outcome measurements are needed to de needed for monitoring nutritional interventions. Promote post-pyloric feeding tube placement practice dictates that guidelines be supported by the when feasible. The following is a summary of some of the ?best the following is a summary of some of the ?best practice? recommendations from the various organiza practice? recommendations from the various organiza tions: tions: Nutritional support should be initiated early within Use of trophic or ?trickle feeding? and permissive the first 24?48 hours in critically ill patients. Indirect calorimetry should be used when available or when predictive equations are known to be inaccu rate. A Guide to the Nutritional Assessment and Treatment of the Critically Ill Patient 2013 37 Clinical Practice Recommendations Table 13. A Guide to the Nutritional Assessment and Treatment of the Critically Ill Patient 2013 39 Summary Nutritional support is important in the care of pa Varying degrees of agreement, disagreement, and tients with acute and chronic illness.
Buy generic viagra plus 400 mg online
Course of ulcerative colitis: analysis of Competing interests Dr Craig Mowat has received support to attend academic changes in disease activity over years erectile dysfunction treatment nj discount viagra plus 400mg without prescription. He has received support to attend academic meetings from Abbott, ulcerative colitis: follow-up of a population-based cohort in Copenhagen County. Toward an integrated clinical, Ferring, and has on-going research support from Novartis and Genentech. He has also received honoraria from Shire, Proctor and Gamble, Gastroenterol 2005;(19 Suppl A):5e36. He has received honoraria from Ferring, Abbott, Schering-Plough, Procter and Gamble, 25. Aliment Pharmacol Ther disease: a prospective comparison of capsule endoscopy with magnetic resonance 2005;21:921e31. Guidelines for management of patients with a short with Pentasa (mesalazine) is superior to oral therapy alone in patients with bowel. Remission induced by an elemental diet induction of remission in ulcerative colitis. Oral beclometasone dipropionate in the effective than twice daily in patients with quiescent ulcerative colitis. Clin treatment of extensive and left-sided active ulcerative colitis: a multicentre Gastroenterol Hepatol 2009;7:762e9. Slow-release 5-amino-salicylic acid (Pentasa) for the treatment for ulcerative colitis. A randomized, double-blind, patients with active extensive and left-sided ulcerative colitis. Oral 5-aminosalicylic acid for maintenance of medically Therapeutique des Affections In? Gastroenterology to budesonide in achieving and maintaining mucosal healing and histologic 2004;126:1733e9. Antibiotic treatment of small bowel postoperative complications in patients with in? Azathioprine intolerance in patients with double-blind, placebo-controlled pilot study. An antibiotic regimen for the treatment and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. A comparison of methotrexate with associated with higher 6-thioguanine levels in adults and children with in? Congenital thiopurine methyltransferase a double-blind, randomized, Israeli multicenter trial. Am J Gastroenterol safety of subcutaneous versus oral administration of methotrexate in patients with 2008;103:1783e800. Increased risk of lymphoma among technical review on the use of gastrointestinal medications in pregnancy. Azathioprine for maintenance of intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Clin long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Azathioprine is useful in Ulcerative Colitis: Outcome and Predictive Factors After Drug Withdrawal. Am J maintaining long-term remission induced by intravenous cyclosporine in steroid Gastroenterol 2009;104:2760e7. Resolution of non-small-cell lung cancer ulcerative colitis: two cases of mucosal healing and clinical response at two years. Long-term outcome of adalimumab moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care therapy for ulcerative colitis with intolerance or lost response to in? Clin Gastroenterol Hepatol moderately severe ulcerative colitis: a randomized, placebo-controlled study. Aliment Pharmacol Ther increase short-term postoperative infectious complications in patients with 2009;29:527e34. N Engl recommendations for assessing risk and for managing Mycobacterium tuberculosis J Med 1987;317:1625e9. Aliment Pharmacol literature on treatment options for left-sided ulcerative colitis and ulcerative Ther 2008;27:19e30. Evaluation of the learning curve in rectal mesalamine versus combination therapy in the treatment of distal ulcerative laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Impact of hospital volume on treatment of acute ulcerative proctosigmoiditis: results of a multicentered placebo postoperative morbidity and mortality following a colectomy for ulcerative colitis. Prevalence of proximal faecal stasis in active ulcerative experience and individualized management of leaks after ileal pouch-anal colitis. Fertility is reduced after restorative the prevention, diagnosis and management of opportunistic infections in proctocolectomy with ileal pouch anal anastomosis: a study of 300 patients. Cochrane Database Syst Rev 2000;(2): together with symptom assessment are required to diagnose pouchitis. Pre-pouch ileitis: a disease of the ileum in unselected European cohort followed for 10 years. Subtotal colectomy for severe acute colitis: diagnosis for symptomatic patients with ileal pouch-anal anastomosis. Am J a 20-year experience of a tertiary care center with an aggressive and early surgical Gastroenterol 2002;97:972e7. Ann R Coll Surg Engl 2008;90: treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Four-week open-label trial of metronidazole restorative proctocolectomy: rationale for a surgical strategy in ulcerative colitis. Medical therapy for induction and proctocolectomy with or without defunctioning ileostomy. Oral budesonide in the treatment of stapled double lumen pouch and the sutured quadruple pouch for restorative chronic refractory pouchitis. Quality of life before and after proctocolectomy and refractory distal ulcerative colitis and ?pouchitis. The ileoanal pouch procedure in the long after ileal pouch-anal anastomosis for chronic ulcerative colitis. Avoidance of laparotomy for recurrent disease is a long well tolerated in patients with in? Azathioprine and 6 disease: follow-up of a population-based cohort in Copenhagen County, Denmark. Long-term surgical recurrence, screening and surveillance in moderate and high risk groups (update from 2002). Safety of immunomodulators and biologics for the treatment of sucrose for patients with in? Neuropharmacology of stress-induced treatment of primary sclerosing cholangitis is safe and effective. Instruct patients to treatment of: seek medical advice if signs or symptoms of clinically important. If a serious infection develops, psoriasis who are candidates for systemic therapy or phototherapy. The recommended dose is 160 mg (two 80 mg injections) at Week 0, followed by 80 mg at Weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks. The recommended dose in pediatric patients from 6 to less than 18 years of age with moderate-to-severe plaque psoriasis is based on the following weight categories. For psoriatic arthritis patients with coexistent moderate-to-severe plaque psoriasis, use the dosing regimen for adult plaque psoriasis [see Dosage and Administration (2. Do not use if the liquid contains visible particles, is discolored or cloudy (other than clear and colorless to slightly yellow). Administer each injection at a different anatomic location (such as upper arms, thighs or any quadrant of abdomen) than the previous injection, and not into areas where the skin is tender, bruised, erythematous, indurated or affected by psoriasis. A similar increase in risk of infection was seen in placebo-controlled trials in patients with pediatric psoriasis, psoriatic arthritis and ankylosing spondylitis [see Adverse Reactions (6. Specific Adverse Drug Reactions: Injection Site Reactions the most frequent injection site reactions were erythema and pain. Active Comparator Trials In the two clinical trials that included an active comparator, the rate of serious adverse events during weeks zero to twelve was 0. The assay to test for neutralizing antibodies has limitations detecting neutralizing antibodies in the presence of ixekizumab; therefore, the incidence of neutralizing antibodies development could be underestimated.

